Invitro Antifungal Evaluation of Denture Soft Liner Incorporated with Tea Tree Oil: A New Therapeutic Approach Towards Denture Stomatitis
Koteswara Rao Pachava1, Lakshmi Kavitha Nadendla2, Leela Subhashini Choudary Alluri3, Huma Tahseen4, Navya Poojitha Sajja5
1 Senior Lecturer, Department of Prosthodontics, Kamineni Institute of Dental Sciences, Sreepuram, Narketpally, Nalgonda District, Telangana, India.
2 Reader, Department of Oral Medicine and Radiology, Kamineni Institute of Dental Sciences, Sreepuram, Narketpally, Nalgonda District, Telangana, India.
3 Assistant Dentist, Kamineni Institute of Dental Sciences, Sreepuram, Narketpally, Nalgonda District, Telangana, India.
4 Assistant Dentist, Kamineni Institute of Dental Sciences, Sreepuram, Narketpally, Nalgonda District, Telangana, India.
5 Intern Student, Kamineni Institute of Dental Sciences, Sreepuram, Narketpally, Nalgonda District, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Koteswara Rao Pachava, Senior Lecturer, Department of Prosthodontics, Kamineni Institute of Dental Sciences, Sreepuram, Narketpally, Nalgonda District, Telangana-508254, India.
E-mail: koteswar_pachava@rediffmail.com
Aim
Adherence and colonization of candida on denture soft liners is the most important contributing factor in development of denture stomatitis. This invitro study is undertaken to investigate whether the incorporation of tea tree oil into denture soft liners would inhibit the growth of candida albicans.
Materials and Methods
Each 10 specimen disks incorporated with tea tree oil into soft liners (St) and without tea tree oil (S) were prepared. Both the tea tree oil daily. These disks were inoculated with candida albicans suspension for assessment of fungal growth and were rinsed with sterile water to remove loosely attached surface organisms. The attached yeasts were measured by inoculating them on saboraud’s agar. Treated and control disks were stored in distilled water for 1, 30, 60 days and washed daily with wet cotton. Data between treated and control disks were compared by applying t-test.
Results
The mean colony forming units (CFU) per mm2 for specimens without tea tree oil after water storage and wash with wet cotton for 1, 30 and 60 days was 7.1 × 106, 6.5 × 106, 6.8 × 106, respectively and for specimens with tea tree oil CFU decreased significantly to 2.1 × 106, 2.8 × 106, 3.1 × 106 after 1, 30 and 60 days. Treated disks were effective in controlling the growth of C.albicans for two months following water storage.
Conclusion
Addition of tea tree oil to denture soft liner significantly reduced growth of C.albicans suggesting a new form of intra oral effective antifungal management for denture stomatitis.
Candida albicans, Colony forming units, Inoculum, Melaleuca alternifolia, Sabouraud’s agar, Silicone soft liner
Introduction
Denture soft liners are mainly used for therapeutic purpose in patients who are not able to tolerate denture induced stresses [1]. Soft liner materials, though being used widely as dynamic impression materials and also as adjuncts in prosthodontics for management of traumatized oral mucosa, have few physical and microbiological disadvantages [2]. One such major severe problem is colonization of denture surface by Candida albicans and other micro organisms, thereby causing denture stomatitis [2].
The candida associated denture stomatitis is a common condition in complete denture wearers, characterized by generalized inflammation of the palatal mucosa covered by the denture [3]. It is estimated to affect about 72% of this population [4]. Denture induced stomatitis can be managed by either denture repair or replacement, prophylactic measures adopted by the patients and prescribing antifungal drugs [5–6]. Biofilms of candida on mucosal and inert surfaces such as dentures may contribute to therapeutic failure by modifying the susceptibility to antifungal agents [7]. This treatment is complicated further in early and institutionalized patients with limitation of motor skills and special needs due to factors like loss of memory, difficulty in proper cleaning of the denture and following strict routine application of topical antifungal agent [8]. Poor patient compliance due to need for frequent drug application and associated adverse effects could also result in recurrence of disease [7].
These short comings have stimulated the development of other methods of drug elution, such as the incorporation of antifungal or antimicrobial agents with denture acrylic resin or with soft liners. A method of treatment by combining tissue conditioner and antifungal agents was suggested initially [9]. After that several attempts have been made to incorporate different antifungal agents such as propolis [10], zeolite [11,12], chlorhexidine [3], Fluconazole [3], punica granatum [13], Nystatin [6,14], Itraconazole [6], Miconazole [15], Ketoconazole [15], Clotrimazole [1] in the resilient liners with varying degree of success.
The recent craze in natural health has contributed to the growing interest in commercially available naturopathic remedies. Medicinal plants extracts have been used in developing countries as alternative treatments to health problems. The essential oil of Melaleuca alternifolia, also known as tea tree oil (TTO) is a new multi-purpose herb that can be obtained from its leaves by steam distillation [16]. TTO has been shown to be promising as a topical antifungal agent, with recent clinical data indicating efficacy in the treatment of dandruff and oral candidiasis [17]. The major advantages of natural medicinal plant extracts as antimicrobial agents include enhanced safety and stability without any side effects, which lack with both organic and inorganic antimicrobial agents. This invitro study is undertaken with the aim to test the efficacy of the denture soft liner combined with TTO against Candida albicans growth.
Materials and Methods
The study was conducted in the department of Prosthodontics, Kamineni Institute of Dental Sciences, Telangana state, India after approval by institutional ethics committee. The silicone soft liner selected was GC Reline Extra soft (G-C Dental Industrial Corp. Tokyo, Japan). TTO was bought from local market.
Specimen Preparation
The study consisted of 6 groups of soft liner specimens (each group 10 no.s), among which 3 groups (S1, S30, S60) were with silicone soft liner alone and 3 groups (ST1, ST30, and ST60) were with addition of TTO into liner material as displayed in [Table/Fig-1]. The soft liners were processed according to manufacturer’s directions. The soft liner material was mixed in supplied automix cartridges and the mix was directly placed into the ring form of mould with a diameter of 5mm and 1mm thickness. Thus, all the specimens were prepared to a uniform size with smooth surfaces by placing polyester film over them. ST specimens were prepared by adding 15% concentration of TTO by weight to silicone samples and processed as above. A total of 60 specimens (30 with liner and 30 with TTO added liner) were prepared and allowed for autopolymerization for 20 minutes at room temperature. Later the specimens were stored in distilled water for day 1 (S1, ST1); 30 days (S30, ST30); and 60 days (S60, ST60) and were cleaned with wet cotton gently for one minute each day.
Showing all test groups of silicone soft liner (Each group= 10)
| S1 without TTO, stored in distilled water for 1 day |
| ST1 with TTO, stored in distilled water for 1 day |
| S30 without TTO, stored in distilled water for 30 days |
| ST30 with TTO, stored in distilled water for 30 days |
| S60 without TTO, stored in distilled water for 60 days |
| ST60 with TTO, stored in distilled water for 60 days |
Fungal growth Assessment
Standard ATCC (10231) approved C.albicans strains were collected. Sabouraud’s dextrose agar medium was prepared. Five ml sabouraud’s broth was poured into each test tube and was autoclaved. The broth was inoculated with full loop of C.albicans 24 hours before placing the discs, so that the organisms were in active growth phase when broth was added to disks. The discs were placed on a membrane in a well of Transwell plate with two disks per well after 24 hours.
An inoculum of 107CFU / ml was prepared, and sabouraud’s broth was inoculated into each well with adjusted yeast suspension. Such plates were incubated for 24 hours at room temperature. Growth controls consisting of 1ml of SDB were inoculated for each test. The broth was removed with a sterile pipette after incubation. The disks were rinsed with sterile water to remove the loosely attached C.albicans. Surface organisms were removed from the disks by placing it in sterile test tubes containing sterile saline and sonicating for 5 minutes. Serial dilution (10x) was prepared of the eluate and 100 μl of each eluate was placed on duplicate plates with sabouraud’s agar. The plates were incubated at 370c for 24 hours and the colonies were counted. C.albicans growth assay was carried out for the 6 groups of specimens on day 1(S1, ST1), day 30 (S30, ST30) and day 60 (S60, ST60). Student’s t-test was applied to analyse the data using SPSS software.
Results
Results suggested that there was a significant difference between the mean CFU per mm2 for soft liner with TTO and untreated control liner at each time interval at 1, 30 and 60 days [Table/Fig-2]. Colonization was lower in TTO combined disks [Table/Fig-3] in comparison to control disks (p = 0.001). Statistically no significant difference was found in CFU of control disks following water storage up to 60 days. Growth of C. albicans was significantly inhibited up to 60 days in treated disks following storage in distilled water and washing with wet cotton daily for one minute.
Showing the mean differences of colony forming units per mm2 × 106 between the experimental groups of soft liner
| Specimens | n | Mean ± SD | 95% confidence interval | p-value |
|---|
| S1 | 10 | 7.1 ± 4.2 | 6.9 – 7.5 | 0.001 |
| ST1 | 10 | 2.1 ± 3.6 | 4.5 – 6.2 | |
| S30 | 10 | 6.5 ± 5.3 | 6.3 – 6.9 | 0.001 |
| ST30 | 10 | 2.8 ± 2.5 | 2.1 – 3.0 | |
| S60 | 10 | 6.8 ± 5.4 | 3.5 – 4.7 | 0.001 |
| ST60 | 10 | 3.1 ± 2.4 | 2.9 – 3.4 | |
| Total | 60 | 5.1 ± 1.8 | 4.8 – 5.4 | |
Showing less colony forming units around the well with tea tree oil
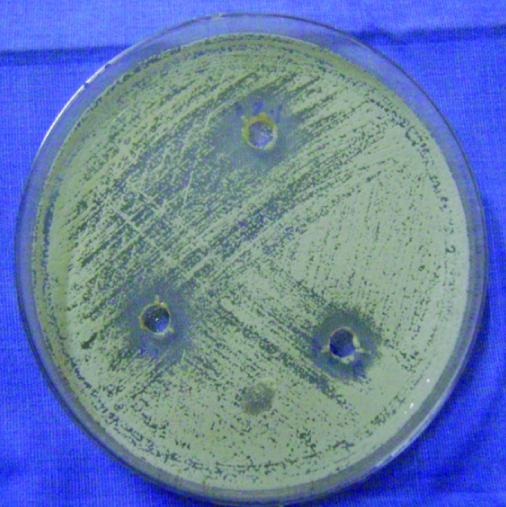
Discussion
Removable prosthesis, when placed in the oral cavity produces numerous changes in the oral environment, which may adversely affect integrity of oral tissues, denture stomatitis being one of the important clinical presentations of oral candidiasis [9]. Though the aetiology is multifactorial [4], denture bio-film components, such as C.albicans play a basic role in development of candidiasis [18]. Newer agents from natural resources are required, which can inhibit the growth of microorganisms in the biofilm, and would enhance the effective alternative therapeutic modalities, as the action of antifungal agents may be limited by their penetration and chemical reaction into biofilm matrix, the extracellular polymeric material [19]. Recently, incorporating extracts of medicinal plants into biomaterials have been in practice and found to be a natural alternative with excellent antifungal effects [16].
TTO, the volatile essential oil from Australian native plant Melaleuca alternifolia, have been largely employed primarily for its antimicrobial and anti inflammatory properties, and shows promise as a topical antifungal agent [20]. This present study incorporated TTO into silicone soft liner and evaluated its efficacy against growth of C. albicans.
Results of present study suggested that TTO treated disks showed significant antifungal efficacy against C. albicans compared to untreated disks upto 60 days, and this was in agreement with Al-Mashhadane et al., [16] showed that 15% TTO had significant antifungal effect against C.albicans on the surface of heat cure acrylic denture base material. This study immersed the denture in TTO for 24–48 hours instead of adding it in the denture itself. Our study has added TTO into the soft liner so that there is continuous sustained release of TTO exhibiting antifungal activity up to 60 days, avoiding other alternative mechanical and chemical denture cleansing methods [21].
This study also supports the results of Hammer et al., [17] suggested that the treatment of C.albicans with TTO exert antifungal action by altering membrane properties of fungal cells, which may alter their permeability and affect the membranes ability to osmo regulate the cells adequately or to exclude toxic materials. Our study results are also in agreement with Emira et al., [22] suggested that plants essential oils significantly prevent the formation of biofilm at low concentrations and the potential bio active compounds in TTO has distinct influence on candida cell growth, function and biofilm formation by interfering any of the steps involved in bio film development and has a potential anti-adhesive effect of candida strains on PMMA.
An invitro study by Merta et al., [23] suggested that the clinical candida strains that are resistant to Fluconazole when exposed to sublethal concentrations of TTO and fluconazole, explored a change in the activity of Fluconazole. TTO enhanced the activity of Fluconazole against resistant strains and it was concluded that TTO can be used as a single therapy or in combination with other conventional drugs like Fluconazole that can be used to treat difficult yeast infections.
Conclusion
Resilient soft liners combined with TTO have shown invitro antifungal efficacy up to 60 days suggesting that the possibility of this essential oil for therapeutic use against denture stomatitis and possibly other oral infections.
[1]. Vojdani M, Zibaei M, Khaledi AAR, Zomorodian K, Ranjbar MA, Boshehri S, In-vitro Study of the Effect of Clotrimazole Incorporation into Silicone Soft Liner on Fungal ColonizationShiraz Univ Dent J 2009 9(Suppl. 1):19-23. [Google Scholar]
[2]. Bal BT, Yauzyilmaz H, Yucel M, A pilot study to evaluate the adhesion of oral microorganisms to temporary soft lining materialsJ Oral Sci 2008 50(1):1-8. [Google Scholar]
[3]. Amin WM, Al – Ali MH, Salim NA, Al – Tarawneh SK, A new form of intra oral delivery of antifungal drugs for the treatment of denture induced oral candidosisEuropean J Dent 2009 3:257-66. [Google Scholar]
[4]. Budtz-Jorgersen E, oral candidiasis in long term hospital care denture wearers with denture stomatitisOral Dis 1996 2(4):285-90. [Google Scholar]
[5]. Muzyka BC, Oral fungal infectionDent Clin North Am 2005 49:49-65. [Google Scholar]
[6]. Chow CKW, Matear DW, Lawrence HP, Efficacy of antifungal agents in tissue conditioners in treating candidiasisGerontology 1999 16:110-19. [Google Scholar]
[7]. Ryalat S, Darwish R, Amin W, New form of administering chlorhexidine for treatment of denture – induced stomatitisTherapeutics and Clinical Risk Management 2011 7:219-25. [Google Scholar]
[8]. Casemiro LA, Martins CHG, Pires-de-Souza FCP, Panzeri H, Antimicrobial and mechanical properties of acrylic resins with incorporated silver – zinc zeolite part-IGerodontology 2008 [Google Scholar]
[9]. Gupta H, Bhat A, Prasad KD, Prasad KMS, Kumar KV, An innovative method of incorporating antifungal agents into tissue conditioners: An invitro studyTrends Biomater Artif Organs 2011 25(2):63-66. [Google Scholar]
[10]. Santos VR, Gomes RT, de Mesquita RA, de Moura MD, França EC, de Aguiar EG, Efficacy of Brazilian propolis gel for the management of denture stomatitis: a pilot studyPhytother Res 2008 22(11):1544-47. [Google Scholar]
[11]. Nikawa H, Yamamoto T, Hamada T, Rahardjo MB, Murata H, Nakanoda S, Antifungal effect of zeolite incorporated tissue conditioner against Candida albicans growth and/or acid productionJ Oral Rehabil 1997 24:350-57. [Google Scholar]
[12]. Jang KS, Inhibitory effect of antifungal agents incorporated in denture lining materials against candida albicansJ Korean Acad Prosthodont 1999 37(3):293-300. [Google Scholar]
[13]. Vasconcelos LC, Sampaio MC, Sampaio FC, Higino JS, Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitisMycoses 2003 46(56):192-96. [Google Scholar]
[14]. Thomas CJ, Nutt GM, The invitro fungicidal properties of Visco-gel, alone and combined with nystatin and amphotericin BJ Oral Rehabil 1978 5:167-72. [Google Scholar]
[15]. Quinn DM, The effectiveness, invitro, of miconazole and ketoconazole combined with tissue conditioners in inhibiting the growth of Candida albicansJ Oral Rehabil 1985 12:177-82. [Google Scholar]
[16]. Al-Mashhadane FAM, Tea tree oil: A new antifungal agents against candida albicans cells on heat cured acrylic resin denture base material. An invitro studyAl – Rafiadain Dent J 2007 7:54-7s. [Google Scholar]
[17]. Hammer KA, Carson CF, Riley TV, Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on candida albicans, candida glabrata and sacchromyces cerevisiaeJ Antimicrobial Chemotherapy 2004 53:1081-85. [Google Scholar]
[18]. Kulak Y, Kazazoglu E, In vivo and invitro study of fungal presence and growth on the three tissue conditioning materials on implant supported complete denture wearersJ Oral Rehabil 1998 25:135-38. [Google Scholar]
[19]. Kanathila H, Bhat AM, Krishna PD, The effectiveness of magnesium oxide combined with tissue conditioners in inhibiting the growth of candida albicans: An invitro studyIndian J Dent Res 2011 22(4):613 [Google Scholar]
[20]. Carson CF, Hammer KA, Riley TV, Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal propertiesClin Microbiol Rev 2006 19(1):50-62. [Google Scholar]
[21]. Yadav R, Yadav VS, Garg S, Mittal S, Garg R, Effectiveness of different denture cleansing methods on removal of biofilms formed in vivoJ Cranio Maxillary Diseases 2013 2(1):22-27. [Google Scholar]
[22]. Emira N, Mejdi S, Aouni M, Invitro activity of Melaleuca alternifolia (Tea tree) and Eucalyptus globules essential oils on oral candida biofilm formation on polymethyl methacrylateJ of Medicinal Plant Research 2013 7(20):1461-66. [Google Scholar]
[23]. Mertas A, Garbusinska A, Szliszka E, Jureczko A, Kowalska M, Krol W, The Influence of Tea Tree Oil (Melaleuca alternifolia) on Fluconazole Activity against Fluconazole-Resistant Candida albicans StrainsBioMed Research International 2015 2015:590470 [Google Scholar]