Introduction
Over the past two decades, molecular pathology techniques have assumed more important roles in the diagnostic work-up of lymphomas [1-3] . In fact, immunohistochemistry (IHC) now forms an integral part of diagnostic haematopathology and is required for the routine diagnosis and classification of virtually all lymphoid malignancies [3,4] .
The review of literature reveals that the information available on the immunohistochemical features of lymphomas in Nigerians falls short of desirable [5-7] . Much of what is known today about lymphomas is the result of research work conducted outside Nigeria and Africa and these may only find limited applications in our local population. This has resulted in poor diagnosis, classification and management outcomes [7] .
Although lymphomas account for approximately 3-4% of cancers worldwide, the incidence rates have been increasing, resulting in significant morbidity and mortality even among African, and especially, Nigerian populations [8-10] . These increases may not be unconnected with the changes being observed in contemporary diagnostic pathology practice in Nigeria which now require more the routine use of advanced techniques like immunohistochemistry among other molecular pathology methods [11,12] . It is expected that this will make the diagnosis and classification of lymphomas easier, more precise and in tandem with the new consensus World Health Organization (WHO) scheme with the attendant positive impact on the management and prognostication of affected patients [13,14] .
It, therefore, becomes imperative to reappraise the biological characteristics of lymphomas in our local population and reclassify these according to the new WHO classification scheme with the aid of appropriate immunohistochemical markers.
Finally, the pathogenetic relationship between EBV and malignant lymphomas in Nigerians has not been clearly defined despite the endemicity of the virus in the population. Nevertheless, various reports seem to suggest that a possible association does exist [15-17] .
Materials and Methods
The demographic data and paraffin blocks from all cases histologically diagnosed as malignant lymphoma in the Department of Morbid Anatomy and Forensic Medicine of the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC) Ile-Ife, Nigeria over a ten year period (June 2002-May 2011) were retrieved and used for this study. Approval was obtained from the local ethics committee of the OAUTHC, Ile-Ife, Nigeria. These were classified according to the WHO scheme using immunohistochemistry [18] .
Immunohistochemical Studies
Immunohistochemistry was performed by the indirect immunoperoxidase method using the tissue microarray (TMA) constructed from representative cores taken from the appropriate formalin-fixed, paraffin-embedded tissue blocks after the original H&E slides were reviewed. The microarray was constructed using a Beecher Instruments arrayer (Beecher Instruments, Wisconsin, USA). The sections were dewaxed and rehydrated while antigen retrieval was performed according to the protocol for the different antigens [Table/Fig-1]. Endogenous peroxidase was blocked by treating the hydrated sections with 3% H202 in methanol for 30 minutes. To prevent non-specific reactions, sections were incubated with 10% serum for 10 minutes. Antigen localization was carried out using TM immunohistochemistry detection system (Biogenex, California, USA). Immunostaining was done using the automated system developed by Biogenex. 3,3’ diaminobenzidine was used to visualize the antigen-antibody reaction. Appropriate positive and negative controls were used. Staining was considered positive if more than 30% of the tumour cells stained with the antibody.
Detection of Epstein-Barr virus
Epstein-Barr virus (EBV) encoded ribonucleic acid (EBER) was detected by in-situ-hybridization (ISH) using an EBV probe-ISH kit according to the manufacturer’s instruction (Novocastra, Newcastle Upon Tyne, UK) following proteinase-K digestion. The hybridization was done for four hours at a temperature of 37oC with fluorescein-conjugated oligonucleotide probes. Probe localization was carried out afterwards using rabbit Fab anti-fluorescein isothiocyanate. 5-bromo-4-chloro-3-indolyl phosphate was used as substrate to produce the blue-black signal. For optimal mRNA preservation in these cases, a poly-dT probe on a seperate section of the paraffin block was used as control. Appropriate controls were used in each case.
Statistical Analysis
The data were analysed using SPSS version 16.0 (p≤0.05) by SPSS version 16 for Windows. The level of statistical significance was set at p <0.05. Cases with incomplete biodata, missing or inadequate archival tissue material were excluded from the study.
Results
A total of 96 cases were identified and retrieved for this study. Of these only 83 cases were proven to be lymphomas using appropriate immunohistochemical markers. The 13 cases excluded from the study included 8 cases with inadequate tissue blocks and 5 cases ultimately diagnosed as non-lymphoid malignancies.
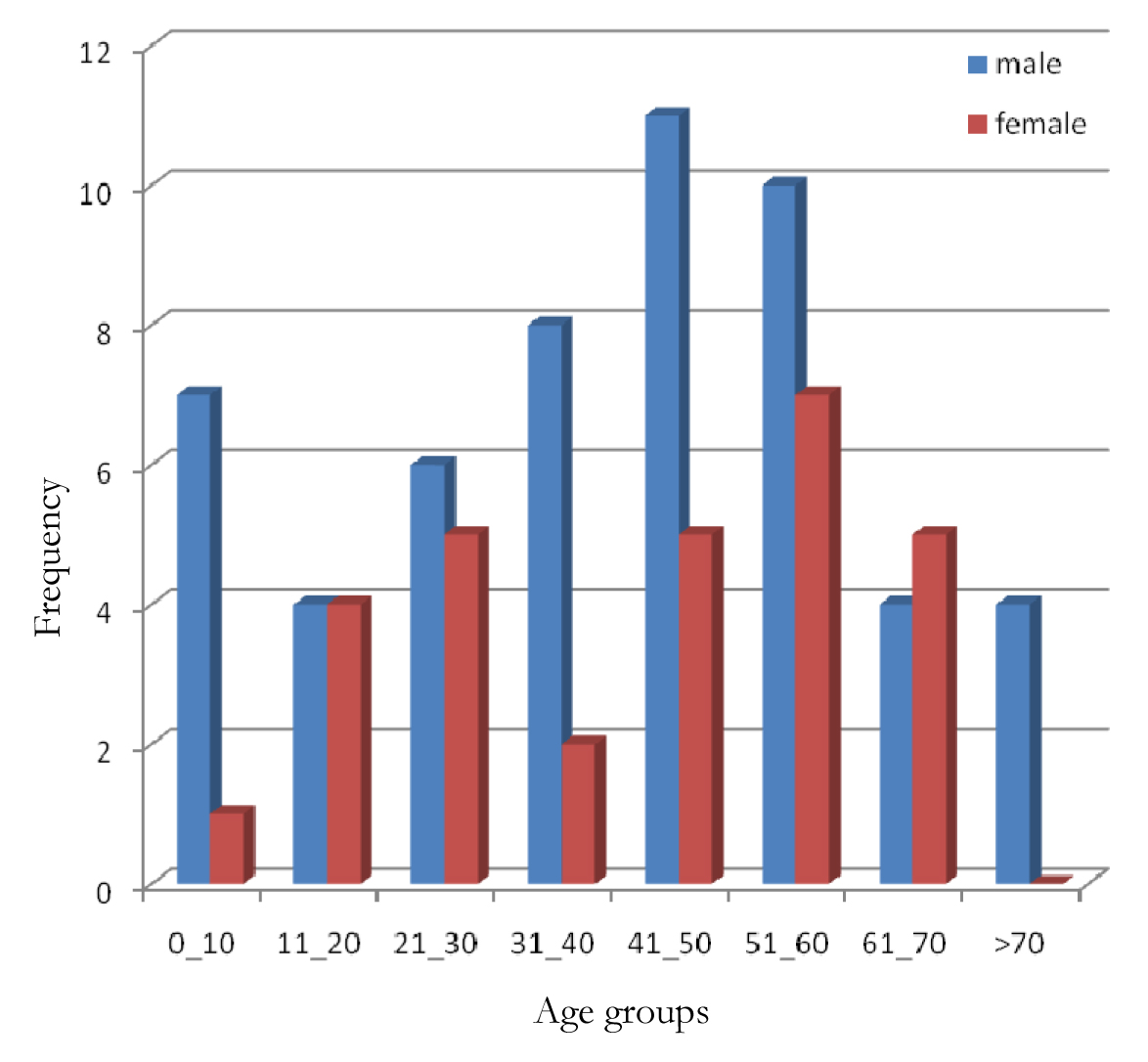
Of the 83 cases thus classified there were 54 (65.1%) males and 29 (34.9%) females with an overall male to female ratio of 1.9:1 [Table/Fig-2]. The overall mean age was 41.7 ± 21.0 years while the ages ranged from 4-96 years. The mean age for males was 41.3 years while that for females was 42.9 years. About 54 (65.1%) cases were found between the ages of 21 and 60 years with only 16 (19.3%) and 13 (15.7%) cases below and above the ages of 20 years and 70 years respectively.
Eighty-two (98.8%) cases were B-cell lymphomas while there was only 1 (1.2%) case of T cell lymphoma [Table/Fig-3]. Seventeen (20.5%) cases were classified as Hodgkin lymphomas while 66 (79.5%) were categorized as Non-Hodgkin lymphomas. The tumours were primarily nodal in 67 cases. Of the 16 cases that presented at extranodal sites, 6(37.5%) primarily affected the gastrointestinal tract (GIT) while 3 (18.8%) were intra-abdominal but not related to the GIT. Two (12.5%) cases each presented primarily as splenic and jaw masses one case each was retroperitoneal, intraorbital and sinonasal.
According to [Table/Fig-3], only 9 subtypes of lymphomas were identified in this study. Their histopathological features and immunohistochemical profiles are summarized in [Table/Fig-4]. A summary of their salient features is given below:
Hodgkin Lymphoma
There were 17 cases of Hodgkin lymphoma and all were found to be of the classical type (CHL). These formed about 20.5% of all lymphoma cases. It was about 1.8 times more common in males than in females. It was found in all age groups with the highest frequencies occurring among individuals in the 21-40 year age group and those younger than 20 years old with 6 (35.5%) and 5 (29.4%) cases respectively. There were only 2 (11.8%) cases older than 60 years old. [Table/Fig-5a-c] shows photomicrographs of one case of CHL.
Diffuse Large B-Cell Lymphoma
Diffuse large B-cell lymphomas (DLBCL) formed the largest group with about 39 (47.0%) cases. The age range was from 6 to 69 years while the mean age was 45.6 years. It was found in all age groups although it was more prevalent in the 21-60 year age group with about 27 (69.2%) cases. It was 2.5 time more common in males. Diffuse large B-cell lymphomas accounted for about 51.9% of cases in males and 37.9% of female cases. The majority of cases (89.7%) were primarily nodal. The extranodal sites included one case each of an intra-abdominal, retroperitoneal, intraorbital and sinonasal mass. About 53.8% of these tumours were high grade while the remainder were low grade tumours. These lymphomas were further categorized into two recognisable prognostic subtypes based on their expression of germinal centre (CD10, BCL-6) or post-germinal centre (MUM-1) markers. About 22 (56.4%) of these were categorized as germinal centre DLBCL with only 1 (2.6%) case of post-germinal DLBCL and 16 (41.0%) cases which did not fit into any of these prognostic subgroups. [Table/Fig-6a,b] shows the photomicrographs of one of the cases.
Burkitt Lymphoma
There were seven cases of Burkitt lymphoma constituting 8.4% of all lymphoma cases. Six of these were seen in children below the age of 20 years while only one case was seen in an adult aged 54-year-old. It was 2.5 times more common in males. About 4 cases occurred at extranodal sites including two jaw masses and two primary intra-abdominal tumours. The three cases with primary nodal disease were found in the cervical lymph node. [Table/Fig-7a-d] shows the photomicrograph of one of the cases.
Marginal Zone Lymphoma
About 8 cases of marginal zone lymphomas were found in this study. The male to female ratio was 1.7:1. These were only seen in individuals older than 21-year-old being most prevalent (62.5%) in individuals between 40 and 60 years old. The age range was 24 to 63 years and the mean age was 44.8 years. All cases were low grade lymphomas. Seven (87.5%) of these were extranodal with the gastrointestinal tract accounting for six of those cases. There was also a case of splenic marginal zone lymphoma. The only case with primary nodal disease affected the cervical node.
Follicular Lymphoma
There were only three cases of follicular lymphoma, two of which were seen in females. All cases were seen in patients older than 40 years. All cases were low grade and primarily occurring in the cervical lymph nodes [Table/Fig-8a-d].
Small Lymphocytic Lymphoma
Six cases of small lymphocytic lymphoma were seen with five (83.3%) occurring above the age of 40 years. It was twice as common in females as in males. All were low grade lymphomas. About five (83.3%) were primarily nodal in distribution all presenting as cervical lymph node masses.
Anaplastic Large Cell Lymphoma
There was only one case of anaplastic large cell lymphoma which occurred in a 45-year-old woman [Table/Fig-9a-d]. This was a high grade T-cell lymphoma. The tumour cells were strongly positive for CD30 with 80% of them positive for Ki 67, but negative for ALK. Protein by immunohistochemistry. The histomorphology essentially consisted of paracortical infiltration by sheets of large lymphoid cells with poor staining horse-shoe shaped nuclei and multiple nucleoli.
B-Cell Lymphoma Unclassified
There was also only one case of B cell lymphoma which was seen in a 22-year-old man. It was a high grade lymphoma and the primary site was the cervical lymph node. The histomorphological and immunohistochemical features were indeterminate between Burkitt lymphoma and diffuse large B-cell lymphoma.
Plasmablastic Lymphoma
The only case of plasmablastic lymphoma was seen in a 53-year-old HIV positive male who presented with a high grade tumour primary affecting the right inguinal node.
The Detection of Epstein-Barr Virus
The great majority (91.6%) of malignant lymphomas in this study were negative for EBV. Epstein Barr virus encoded RNA was detected in a total of seven cases (8.4%). This included 4 (23.5%) cases of classical Hodgkin lymphoma, 2 (28.6%) cases of Burkitt lymphoma and one case of plasmablastic lymphoma. All EBV positive cases of classical Hodgkin lymphoma were of the mixed cellularity type. The association between EBV positivity and lymphoma subtype was statistically significant (p=0.002).
Reclassified Cases
[Table/Fig-10] shows the list of the five cases reclassified as non-lymphoid malignancies. Four of these were carcinomas while the fifth was diagnosed as alveolar rhabdomyosarcoma. The carcinomas included one case each of lymphoephithelioma, melanoma, undifferentiated and anaplastic carcinoma. Four of these were primarily nodal in distribution. All the carcinomas were seen in middle-aged to elderly individulas while the sarcoma was diagnosed in a nine-year-old child.
Discussion
Although lymphomas constitute an important cause of morbidity and mortality in Nigeria data on their immunohistochemical characteristics remain scanty [9,19] . This clinicopathologic review of lymphomas diagnosed in our hospital, thus, provides the first data on their immunohistochemical characteristics in our local Nigerian population.
The overall mean age of 41.7 years in this study compares with what has been reported in various parts of Nigeria and around the world [9,20] . Our study also showed that the disease is twice as common in males as it is in females. This contrasts sharply with those from Europe and North America which show nearly equal sex distribution [21-24] . This perhaps suggests that possible differences may exist between both sexes in the pathogenesis of these tumours in our local population.
Our study identified only nine subtypes of lymphomas, eight of which were non-Hodgkin lymphomas. Overall, intermediate and high grade subtypes predominated unlike in Western countries where low grade lymphomas are more common [1,6,21,23-26] .
The most frequent low grade lymphomas from this study were marginal zone lymphoma, small lymphocytic lymphomas and follicular lymphomas. The marginal zone lymphomas were predominantly extranodal unlike the majority of SLL which presented as cervical lymph node masses. The rarity of follicular lymphoma in Africans was demonstrated in this study [22] . Similarly, our study also confirms the rarity of anaplastic large cell lymphoma and plasmablastic lymphoma as there was only one case of each lymphoma subtype.
Diffuse large B-cell lymphomas formed the vast majority of our cases. These were either intermediate or high grade lesions in nearly equal distribution. Although, they were widely distributed among all age groups, more than two-thirds occurred in individuals older than 20 years old. This is similar to various reports from around the world and Nigeria [9,23,26,27] . It is well known that DLBCL’s are a heterogenous group of tumours with variable morphology, immunophenotype and molecular cytogenetics. They may also represent the endpoint of several indolent lymphomas and therefore could vary widely in incidence and clinical course even in the same region. A large proportion of our cases were sub-classified as belonging to the germinal centre subtype of DLBCL which is typically associated with a favourable prognosis [27,28] . There were, however, about 16 cases which could not be classified into any of the prognostic subgroups based solely on their immunohistochemical profile. Molecular studies will be valuable in unravelling these.
Burkitt lymphoma was the most common childhood lymphoma we encountered in this study. Its frequency was, however, much lower than what has been reported in other series [1,6,15] . This may be due to the fact that majority of cases are diagnosed by the haematologists and do not get to our histopathology department [15] . In addition, there is a reported decline in the incidence of this tumour in our country associated with improvements in our general living conditions [29] .
This study confirms the rarity of nodular lymphocyte predominance Hodgkin lymphoma in Nigerians [6,7,15,16,21] . The predominance of the mixed cellularity variant in this study may not be unconnected with its well known association with the EBV [16] . Just like a previous study from our centre has shown, only the mixed cellularity variant was associated with the EBV, an endemic DNA virus which has been incriminated in the aetiopathogenesis of several malignant B cell lymphomas especially in immunocompromised individuals even in Nigeria [16,17] . Although EBV infection is known to be endemic in Nigeria, its role in the pathogenesis of malignant lymphomas has not been clearly defined. Many reports from Nigeria only provide circumstantial evidence that suggests that such a link does exist. As demonstrated in this study as well as previous such studies from other parts of the country, the implicated lymphoma subtypes include endemic BL, CHL and B cell lymphomas associated with immunosuppression such as HIVassociated lymphomas, plasmablastic lymphomas and many posttransplant lymphoproliferative disorders [16,17,29]. Obviously there is need for a larger multi-centre study to confirm and clearly define the association of EBV with the various subtypes of lymphomas in Nigerians.
The importance of the routine use of immunohistochemistry in the diagnosis of malignant lymphomas even in resource poor countries like Nigeria is underscored by the reclassification of five cases in this study as non-lymphoid malignancies. It therefore becomes imperative that concerted efforts be made to ensure that immunohistochemical techniques are incorporated into the routine diagnostic workup for lymphomas.
List of antibodies, the dilutions and the antigen retrieval protocols used
| ANTIBODY | DILUTION | ANTIGEN RETRIEVAL | SOURCE |
|---|
| CD3 | 1:50 | 20MINS EDTA, PH 8.0 | NOVOCASTRA |
| CD5 | 1:50 | 20MINS EDTA, PH 8.0 | NOVOCASTRA |
| CD4 | 1:400 | 20MINS EDTA, PH 8.0 | NOVOCASTRA |
| CD8 | 1:50 | 20MINS EDTA, PH 8.0 | NOVOCASTRA |
| CD10 | 1:20 | 20MINS EDTA, PH 8.0 | NOVOCASTRA |
| CD15 | 1:10 | 20MINS MW, CITRATE, PH 6.0 | DAKO |
| CD20 | 1:250 | 20MINS MW, CITRATE, PH 6.0 | DAKO |
| CD23 | 1:100 | 20 MINS MW, CITRATE, PH 6.0 | NOVOCASTRA |
| CD30 | 1:50 | 20 MINS DAKO RETRIEVAL SOLUTION, PH 6.0 | DAKO |
| CD45(LCA) | 1:10 | 20 MINS EDTA, PH 8.0 | DAKO |
| CD138 | 1:100 | 20 MINS MW, CITRATE, PH 6.0 | SEROTEC |
| CD79a | 1:20 | 20 MINS MW, CITRATE, PH 6.0 | DAKO |
| BCL-2 | 1:7 | 20 MINS MW, CITRATE, PH 6.0 | DAKO |
| BCL-6 | 1:20 | 20 MINS EDTA, PH 8.0 | DAKO |
| CYCLIN D1 | 1:50 | 20 MINS MW, CITRATE, PH 6.0 | DAKO |
| ALK | 1:10 | 20 MINS DAKO RETRIEVAL SOLUTION, PH 9.9 | DAKO |
| MUM-1 | 1:200 | 20 MINS MW, CITRATE, PH 6.0 | SANTA CRUZ |
| HHV-8 | 1:50 | 20 MINS MW, CITRATE, PH 6.0 | NOVOCASTRA |
| PANKERATIN | 1:150 | 10 MINS TRYPSIN | DAKO |
| Ki-67 | 1:50 | 20 MINS MW, CITRATE, PH 6.0 | NOVOCASTRA |
| MELAN A | 1:50 | 20 MINS MW, CITRATE, PH 6.0. | NOVOCASTRA |
Age and sex distribution of cases of malignant lymphoma
Some clinicopathologic parameters of the various subtypes of malignant lymphoma
| Lymphoma subtype | CHL | DLBCL | BL | MZL | FL | SLL | ALCL | BCLU | PL | Total% |
|---|
| Number (%) | | 17 (20.5) | 39 (47.0) | 7 (8.4) | 8 (9.6) | 3 (3.6) | 6 (7.2) | 1 (1.2) | 1 (1.2) | 1 (1.2) | 83 (100.0) |
| M:F (p=0.448) | | 1.8:1 | 2.5:1 | 2.5:1 | 1.7:1 | 1:2 | 1:2 | All Female | All Male | All Male | 1.9:1 |
Age range (yrs)
Mean age (yrs)
| | 7-73
34.2
| 6-96
45.6
| 4-54
16.9
| 24-63
44.8
| 50-67
57.3
| 40-68
56.0
| Not applicable | Not applicable | Not applicable | 4-96
41.7
|
Age distribution
(years) (p=0.190)
| 0-20
21-40 41-60 61>
| 5 (29.4)
6 (35.3) 4 (23.5) 2 (11.8)
| 5 (12.8)
11 (28.2) 16 (41.0) 7 (17.9)
| 6 (85.7)
0 (0) 1 (14.3) 0 (0)
| 0 (0)
2 (25.0) 5 (62.5) 1 (12.5)
| 0 (0)
0 (0) 2 (66.7) 1 (33.3)
| 0 (0)
1 (16.7) 3 (50.0) 2 (33.3)
| 0 (0)
0 (0) 1 (100.0) 0 (0)
| 0 (0)
1 (100.0) 0 (0) 0 (0)
| 0 (0)
0 (0) 1 (100.0) 0 (0)
| 16 (19.3)
21 (25.3) 33 (39.8) 13 (15.7)
|
| Nodal site(%) | | 17(25.4) | 35(89.7) | 3(4.5) | 1(1.5) | 3(4.5) | 5(7.5) | 1(1.5) | 1(1.5) | 1(1.5) | 67(100.0) |
| Extranodal site (p<0.001) | GIT
Spleen Intraabdominal Jaw Retroperitoneal Intraorbital Sinonasal Total(%)
| -
- - - - - - -
| -
- 1 - 1 1 1 4(25.0)
| -
- 2 2 - - - 4(25.0)
| 6
1 - - - - - 7(43.8)
| -
- - - - - - 0(0.0)
| -
1 - - - - - 1(6.3)
| -
- - - - - - 0(0.0)
| -
- - - - - - 0(0.0)
| -
- - - - - - 0(0.0)
| 6(37.5)
2(12.5) 3(18.8) 2(12.5) 1(6.3) 1(6.3) 1(6.3) 16(100.0
|
| Tumour Grade (p<0.001) | Low(%)
Intermediate(%) High(%) Total(%)
| -
- - -
| 0 (0.0)
18 (46.2) 21 (53.8) 39(100.0)
| 0 (0.0)
0 (0) 7 (100.0) 7(100.0)
| 8 (100.0)
0 (0) 0 (0) 8(100.0)
| 3 (100.0)
0 (0) 0 (0) 3(100.0)
| 6 (100.0)
0 (0) 0 (0) 6(100.0)
| 0 (0)
0 (0) 1 (100.0) 1(100.0)
| 0 (0)
0 (0) 1 (100.0) 1(100.0)
| 0 (0)
0 (0) 1 (100.0) 1(100.0)
| 17 (25.8)
18 (27.3) 31 (47.0) 66(100.0)
|
CHL= Classical Hodgkin Lymphoma; DLBCL= Diffuse large B-cell lymphoma; BL= Burkitt Lymhpoma; MZL= Marginal zone lymphoma; FL= Follicular lymphoma; SLL= Small lymphocytic lymphoma; ALCL= Anaplastic large cell lymphoma; BCLU= B-cell lymphoma unclassified; PL= Plasmablastic lymphoma
Histopathological features and immunohistochemical profiles of the various subtypes of malignant lymphoma
| Lymphoma subtypes | Histopathological features | Positive IHC stains |
|---|
| CHL | Various patterns were observed. Common to all, however, is the presence of a few neoplastic Reed-Sternberg (RS) cells in a mixed reactive inflammatory background of eosinophils, lymphocyets, histiocytes and neutrophils. | CD15, CD30 positive RS cells |
| DLBCL | Diffuse proliferation of large lymphoid cells with vesicular nuclei and prominent nucleoli, and relatively scant cytoplasm. | CD10, CD19, CD20 |
| BL | Diffuse growth of medium sized round lymphoid cells with round to oval nuclei and several prominent basophilic nucleoli. The cytoplasm is amphophilic. Numerous mitotic figures are present. The cells are admixed with tangible body macrophages giving a starry sky pattern | CD10,CD20, BCL6, EBER, Ki-67 >95% |
| MZL | An admixture of small round lymphocytes, monocytoid B cells, cells with slightly irregular nuclei, plasmacytoid cells, plasma cells. | CD20 (negative for CD5, CD23, & CYCLIN D1) |
| FL | Nodular growth pattern characterized by a mixture of different proportions of small and large lymphoid cells. Small cells with scanty cytoplasm and irregular elongated cleaved nuclei and prominent indentations admixed with large cells with vesicular nuclei, one or more nucleoli and a thin rim of cytoplasm. | CD20, BCL-2 (negative for CD5 & CD45) |
| SLL | Monotonous proliferation of small round lymphocytes with clumped nuclear chromatin, scant cytoplasm and scanty mitotic activity. Presence of variable numbers of larger cells (prolymphocytes) with vesicular nuclei and distinct nucleoli, singly and in small aggregates. | CD5, CD23 |
| ALCL | Polymorphic infiltrate of a variable admixture of neutrophils, lymphocytes, histiocytes and large neoplastic cells showing marked pleomorphism. The large cells have multilobed or horseshoe nuclei with prominent nucleoli and abundant eosinophilic cytoplasm. | CD30 |
| BCLU | Features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma | CD10, CD20 |
| PL | Monomorphic population of large cells with vesicular nuclei, single centrally placed prominent nucleolus, abundant basophilic cytoplasm and paranuclear hoff. Presence of immature plasma cells noted | CD38, CD138, MUM-1 (negative for CD20) |
Photomicrographs of Classical Hodgkin’s lymphoma showing: (A) The histological features on haematoxylin and eosin (X400), (B) CD 30 positive Reed-Sternberg cells (X40), (C) Reed-Sternberg cells with nuclear staining for EBV encoded RNA

Photomicrographs of diffuse large B-cell lymphoma showing: (A) The histological features on routine haematoxylin and eosin (X100) (B) Diffuse CD 20 expression pattern (X60)

Photomicrograph of Burkitt Lymphoma showing: (A) The histological features on haematoxylin and eosin (X40), (B) The typical ‘starry-sky appearance on Giemsa (X40), (C) CD 20 expression pattern (X40), (D) Tumour cells with nuclear staining for EBV encoded RNA (X80)
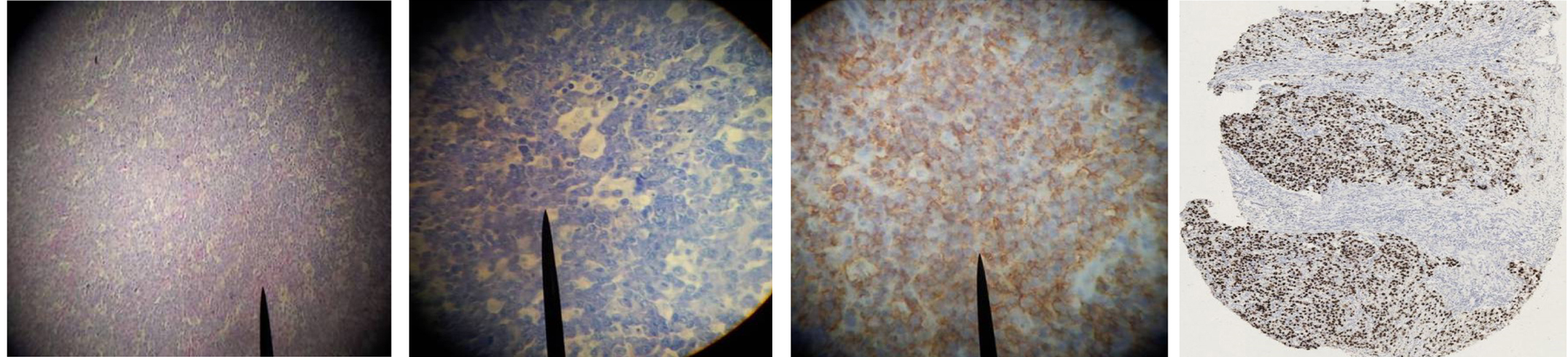
Photomicrograph of follicular lymphoma showing: (A) The nodular growth pattern on haematoxylin and eosin (X40), (B) CD 20 expression pattern (X40), (C) BCL-2 expression (X40), (D) Ki-67 expression (X40)
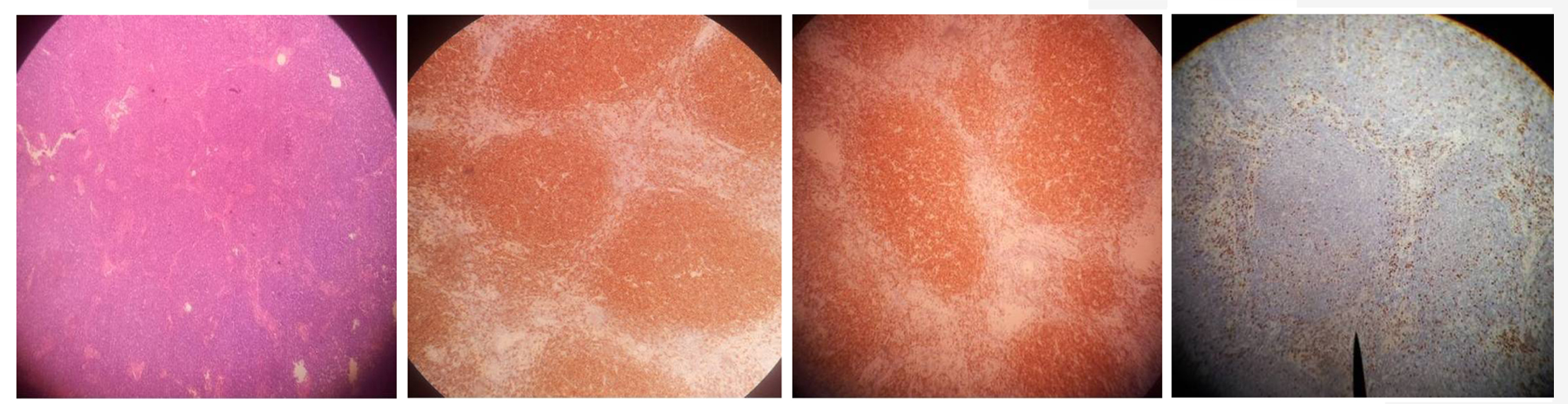
Photomicrograph of anaplastic large cell lymphoma showing: (A) The histological features on routine haematoxylin and eosin (X100), (B) Lack of CD 20 expression (X100), (C) CD 30 expression (X100), (D) Ki-67 index (X100)

Cases reclassified as non-lymphoid malignant disorders
| Age (yrs) | Sex | Biopsy Site | Previous histologic diagnosis | Final diagnosis after immunohistochemistry |
|---|
| 1 | 9 | Female | Cervical lymph node | NHL | Alveolar rhabdomyosarcoma |
| 2 | 52 | Male | Inguinal lymph node | NHL | Melanoma |
| 3 | 57 | Female | Nasopharyngeal mass | NHL | Lymphoepithelioma |
| 4 | 61 | Male | Cervical lymph node | NHL | Undifferentiated carcinoma |
| 5 | 70 | Male | Cervical lymph node | HL | Anaplastic carcinoma |
p=0.218
Conclusion
Immunohistochemistry is vital to the proper classification of lymphomas even in a resource poor environment like Nigeria. Although nine subtypes of lymphomas were identified, B-cell and indeed diffuse large B-cell lymphomas formed the majority of cases. Males were more commonly affected than females. Epstein-Barr virus probably plays an important role in the pathogenesis of malignant lymphomas in our environment although a larger multicentre study is required to prove this.
CHL= Classical Hodgkin Lymphoma; DLBCL= Diffuse large B-cell lymphoma; BL= Burkitt Lymhpoma; MZL= Marginal zone lymphoma; FL= Follicular lymphoma; SLL= Small lymphocytic lymphoma; ALCL= Anaplastic large cell lymphoma; BCLU= B-cell lymphoma unclassified; PL= Plasmablastic lymphoma
p=0.218
[1]. ES Jaffe, NL Harris, H Stein, PG Isaacson, Classification of lymphoid neoplasms: the microscope as a tool for disease discoveryBlood 2008 112(12):4384-99. [Google Scholar]
[2]. P Kluin, E Schuuring, Molecular cytogenetics of lymphoma: where do we stand in 2010?Histopathology 2011 58(1):128-44. [Google Scholar]
[3]. RA Higgins, JE Blankenship, MC Kinney, The application of immunohistochemistry in the diagnosis of non-Hodgkin and Hodgkin lymphomaArch Pathol Lab Med 2008 132(3):441-61. [Google Scholar]
[4]. J Parsonnet, S Hansen, L Rodriguez, AB Gelb, RA Warnke, E Jellum, Helicobacter pylori infection and gastric lymphomaN Engl J Med 1994 330(18):1267-71. [Google Scholar]
[5]. S Lazzi, F Ferrari, OA Nyang, N Palummo, A de Milito, M Zazzi, HIV associated malignant lymphoma in Kenya (Equatorial Africa)Hum Pathol 1998 29(11):1285-89. [Google Scholar]
[6]. KA Adelusola, KA Adeniji, GO Somotun, Lymphoma in adult NigeriansWest Afr J Med 2001 20(2):123-26. [Google Scholar]
[7]. O Oluwasola, JA Olaniyi, JA Otegbayo, GO Ogun, TS Akingbola, CO Ukah, A fifteen-year review of lymphomas in a Nigerian tertiary healthcare centreJ Health Popul Nutr 2011 29(4):310-16. [Google Scholar]
[8]. J Ferlay, H Shin, F Bray, D Forman, C Mathers, MD Parkin, Estimates of world- wide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer 2010 127(12):2893-917. [Google Scholar]
[9]. CE Omoti, NK Halim, Adult lymphomas in Edo state, Niger delta region of Nigeria- Clinicopathologic profile of 205 casesClin Lab Haematol. 2005 27(5):302-06. [Google Scholar]
[10]. D Baris, SH Zahm, Epidemiology of lymphomasCurr Opin oncol 2000 12(5):383-94. [Google Scholar]
[11]. S Gopal, WA Wood, SJ Lee, TC Shea, KN Naresh, PN Kazembe, Meeting the challenge of hematologic malignancies in sub-Saharan AfricaBlood 2012 119(2):5078-87. [Google Scholar]
[12]. EEU Akang, Recent methods and techniques in diagnostic histopathology: Impact on tropical pathology practiceAnnals of Tropical Pathology 2010 1(1):7-16. [Google Scholar]
[13]. E Campo, SH Swerdlow, NL Harris, S Pireli, H Stein, ES Jaffe, The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applicationsBlood 2011 117(19):5019-32. [Google Scholar]
[14]. CH Dunphy, Applications of flow cytometry and immunohistochemistry to diagnostic HaematopathologyArch Pathol Lab Med 2004 128:1004-22. [Google Scholar]
[15]. KA Adelusola, DO Sabageh, CO Ukah, Lymphoreticular diseases in NigerianAfr Health Sci 2008 8(1):20-24. [Google Scholar]
[16]. KA Adelusola, NA Titiloye, MA Durosinmi, O Rotimi, Epstein Barr Virus Latent Membrane Protein-1 in Hodgkin’s Lymphoma in NigeriansAfr Health Sci 2009 9(3):174-78. [Google Scholar]
[17]. Y Iliyasu, LW Ayers, AA Liman, GD Waziri, SM Shehu, Epstein-Barr virus association with malignant lymphoma subgroups in Zaria, NigeriaNiger J Surg Sci 2013 23(1):6-8. [Google Scholar]
[18]. SH Swerdlow, E Campo, NL Harris, ES Jaffe, SA Pileri, H Stein, WHO classification of tumours of the haematopoietic and lymphoid tissues 2008 4th EditionLyonIARC Press [Google Scholar]
[19]. DM Parkin, F Bray, J Ferlay, P Pisani, Global cancer statistics, 2002CA Cancer J Clin 2005 55(2):74-108. [Google Scholar]
[20]. L Hartge, L Devesa, PJ Fraumeni, Hodgkin and non-Hodgkin lymphomasCancer Surv. 1994 19(20):423-53. [Google Scholar]
[21]. E Roman, AG Smithn, Epidemiology of LymphomasHistopathology 2011 58:4-14. [Google Scholar]
[22]. CD Cool, MA Bittery, The malignant lymphomas of Kenya: morphology, immunophenotype, and frequency of Epstein-Barr virus in 73 casesHum Pathol 1997 28(9):1026-33. [Google Scholar]
[23]. SC Peh, Host ethnicity influences non-Hodgkin’s lymphoma subtype frequency and Epstein–Barr virus association rate: the experience of a multi-ethnic patient population in MalaysiaHistopathology 2001 38(5):458-65. [Google Scholar]
[24]. CC Anunobi, AA Banjo, FB Abdulkareem, AO Daramola, RO Akinde, EK Abudu, Adult lymphomas in Lagos Nigeria: a fourteen year studyNig Q J Hosp Med 2007 17(2):63-66. [Google Scholar]
[25]. A Arcan, D Dincol, H Akbulut, H Onur, A Demirkazk, F Cay, Clinicopathologic features and prognostic factors of primary extranodal non-Hodgkin’s lymphomas in Turkey Am J Clin Oncol 1999 2(6):587 [Google Scholar]
[26]. NM Almasri, MA Habashneh, HS Khalidi, Lymphoma in Jordan - types and patterns of 111cases classified according to the WHO classification of haematological malignanciesSaudi Med J 2004 25(5):609-14. [Google Scholar]
[27]. L de Leval, NL Harris, Variability in immunophenotype in diffuse large B-cell lymphoma and its clinical relevanceHistopathology 2003 43(6):509-28. [Google Scholar]
[28]. CP Hans, DD Weisenburger, TC Greiner, RD Gascoyne, J Delabie, S Ott, Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarrayBlood 2004 103(1):275-82. [Google Scholar]
[29]. Al Ojesina, EE Akang, KO Ojemakinde, Decline in the frequency of Burkitt lymphoma relative to other childhood malignancies in Ibadan, NigeriaAnn Trop Paediatr 2002 22:159-63. [Google Scholar]