Ataxia-telangiectasia (AT) is a rare autosomal recessive disorder that is characterized by progressive cerebellar ataxia, telangiectasia, immunodeficiency, and a predisposition to leukemia/lymphoma. Here we report a rare case of lymphoma of the tongue accompanied by AT. Tumour extirpation was performed and diffuse large B-cell lymphoma was diagnosed following pathologic examination. A whole-body survey showed no other enlarged lymph nodes or tumour. The female patient then received a modified dosage of COPAD (cyclophosphamide, vinblastine, pirarubicin, and prednisolone) plus rituximab to avoid severe complications. As of follow-up after 3 years and 5 months, she remains in complete remission. Patients showing AT need careful surveillance and long-term continuous follow-up.
Case Report
A female patient had been diagnosed with ataxia-telangiectasia (AT) at 11 months old, and had received infusions of intravenous immunoglobulin (IVIg) every 4 weeks in the Department of Paediatrics of the University Hospital of Medicine, Tokyo Medical and Dental University, because she had low serum levels of IgG with markedly increased IgM. At the age of 3 year, she developed cerebellar ataxia and involuntary movements. At the age of 8 year, in April 2011, she presented to our department with a 1-month history of a slowly growing mass arising in the tongue. She had no history of neck swelling, throat discomfort, fever, night sweats, or weight loss. Her family history revealed that her elder sister exhibited gait disturbance and died of leukemia at the age of 4 year. Clinical examination revealed a 1.2×1.0-cm smooth-surfaced, nodular submucosal mass on the right inferior surface of the tongue [Table/Fig-1]. On palpation, the lesion was hard with clear margins. No hypesthesia of the right side of the tongue was evident. Salivary outflow from the right sublingual caruncle was normal. No lymphadenopathy was detected on physical examination of the cervical region.
A 1.2×1.0-cm submucosal nodular-appearing, smooth-surfaced mass on the right inferior surface of the tongue

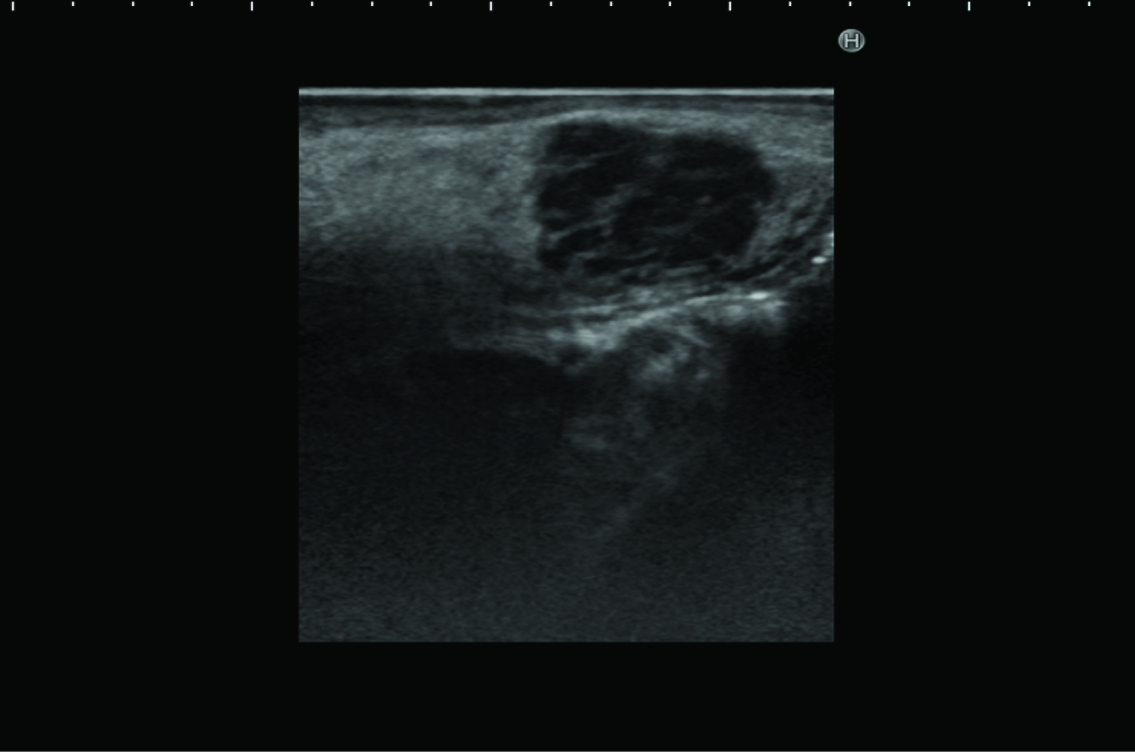
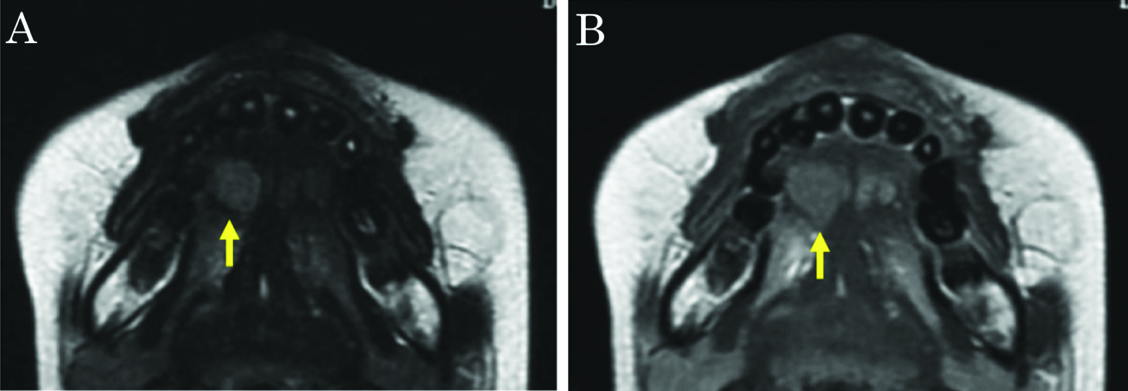
Ultrasonography revealed a rounded mass in the right side of the tongue, with irregular, unsharp borders. Ultrasonography demonstrated a predominantly hypoechoic appearance, with some hyperechoic foci [Table/Fig-2]. Magnetic resonance imaging (MRI) demonstrated a mass in the right side of the tongue, with slightly elevated signal intensity on T2-weighted imaging, and weak enhancement on gadolinium-enhanced T1-weighted imaging [Table/Fig-3a,b]. Laboratory analyses showed: white cell count, 3,000/μl; lactate dehydrogenase, 188 U/l; serum alpha-fetoprotein (AFP), 182 ng/ml; serum IgG, 480 mg/dl; serum IgM, 571 mg/dl; and serum IgA, 4 mg/dl. The tumour was initially diagnosed clinically as salivary gland tumour, such as pleomorphic adenoma. However, we considered that the tumour might represent malignant lymphoma, because the patient had already been diagnosed with AT.
Ultrasonography reveals a rounded mass in the right side of the tongue, with irregular, unsharp borders. The sonogram demonstrates a predominantly hypoechoic appearance, with some hyperechoic foci
A) T2-weighted imaging. The mass showed slightly elevated signal intensity. B) Gadolinium-enhanced T1-weighted imaging. The mass shows weak enhancement
In May 2011, the patient underwent extirpation of the tongue tumour under general anaesthesia, and surgical wound was closed primarily [Table/Fig-4a&b]. Following surgery, neither dysphonia nor dysphagia was identified, and the postoperative course was uneventful.
A) Tumour extirpation of the tongue was carried out under general anaesthesia. B) The surgical wound was closed primarily

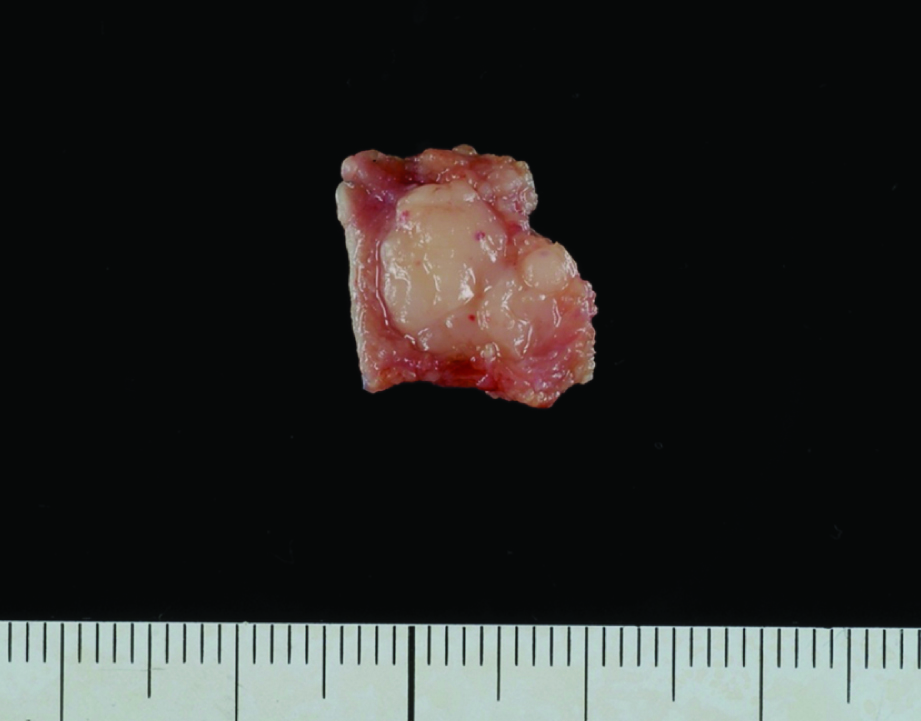
The cut surface was yellowish-white colour, solid and swollen [Table/Fig-5]. Histological examination demonstrated diffuse proliferation of malignant round cells [Table/Fig-6a]. A higher-power view showed that the malignant round cells were large lymphoid cells with hyperchromatic nuclei. Mitotic figures were scattered [Table/Fig-6b]. Immunohistochemical staining of the atypical lymphoid cells showed positive staining for CD20 and CD79-alpha [Table/Fig-6c&d]. The histopathological diagnosis was diffuse large B-cell lymphoma (DLBCL).
The cut surface was yellowish-white colour, solid and swollen
A) Diffuse proliferation of malignant round cells 20x. B) Large lymphoid cells with hyperchromatic nuclei. Scattered mitotic figures are present 400x. C, D) Immunohistochemical examination shows atypical lymphoid cells staining positively for CD20 and CD79-alpha 400x

The patient was admitted to the Department of Paediatrics in the University Hospital of Medicine, Tokyo Medical and Dental University. Examination of bone marrow aspirate examination showed negative results for tumour involvement. MRI was performed as part of a staging work-up and no other lesions were identified. Chemotherapy was considered and, to avoid the severe complications reported in AT patients, a modified dosage of COPAD (cyclophosphamide, vinblastine, pirarubicin, and prednisolone) was chosen. She received cyclophosphamide at 500 mg/m2 on days 1-3, vinblastine at 6 mg/m2 on days 1 and 6, pirarubicin at 20 mg/m2 on days 1 and 2, and prednisolone at 60 mg/m2/ on days 1-6. This treatment was repeated every 4 weeks for 2 cycles. On day 0 of each cycle, rituximab at a dosage of 375 mg/m2 was added. Radiotherapy was not employed. Although grade 4 neutropenia was observed on day 9 of each cycle, supportive therapy with granulocyte colony-stimulating factor at 100 μg/day proved useful for the continuity of treatment. Grade 3 stomatitis, diarrhea, hypoalbuminemia, and hyponatremia were observed, and then the patient underwent administration of albumin and electrolyte fluid management. At follow-up 3 years and 5 months postoperatively, the patient remained in complete clinical remission [Table/Fig-7].
At follow-up at 3 years and 5 months postoperatively, the patient remained in complete clinical remission

Discussion
Ataxia-telangiectasia (AT) is a rare autosomal recessive disorder characterized by progressive cerebellar ataxia, oculocutaneous telangiectasia, recurrent maxillary sinus and lung infections, immunodeficiency, and a predisposition toward cancers, particularly of the lymphoid system [1]. The estimated incidence of AT is 1 in 20,000-300,000 live births. Approximately, one-third of AT patients develop neoplasms during their lifetime, more frequently between 9 and 14 years of age, and most of which (85%) are accounted for by lymphomas and leukemias. AT is caused by germline inactivation of both copies of the ataxia-telangiectasia mutated (ATM) gene at chromosomal region 11q22.3-q23.1. ATM encodes a 350-kDa nuclear phosphoprotein involved in cellular responses to radiation-induced double-stranded breaks in DNA, apoptosis, and cell cycle control [2]. The increased incidence of lymphoid tumours in AT patients suggests that ATM acts as a tumour-suppressor gene. AT is a rare genetic disorder with multiple manifestations. The condition is characterized by early onset of progressive neurodegeneration, oculocutaneous telangectasia, various immunodeficiencies leading to a predisposition to infection, high risk of developing lymphoreticular malignancy, and increased sensitivity to ionizing radiation. Characteristic laboratory and diagnostic findings include cerebellar atrophy, high serum levels of AFP, and chromosomal translocations involving immunoglobulin and T-cell receptor genes. Following cloning of a gene responsible for AT, a diagnosis of AT can be confirmed by measuring ATM protein levels or ATM kinase activity, or by sequencing the ATM gene.
AT is difficult to treat and shows a poor prognosis because of its multisystem involvement. The two major causes of death among AT patients are progressive pulmonary disease and neoplasia [3]. Therapy is primarily supportive, directed at preventing infection with IVIg and prophylactic antibiotics. AT patients are susceptible to damage not only from ionizing radiation, but also from chemotherapic agents. Both cause double-stranded breaks in DNA. AT patients have a reduced tolerance to chemotherapy and an increased risk of life-threatening infections. Treatment of lymphomas in children with AT can thus prove particularly challenging.
Although lymphomas represent the third most common group of malignant lesions of the oral cavity, following squamous cell carcinomas and salivary gland neoplasms, the incidence is only 3-5% [4]. Primary lymphoma of the oral cavity accounts for only 1% of all lymphomas and 2-12% of extranodal lymphomas. The most frequent intraoral sites for lymphoma are the hard palate and gingiva. Non-Hodgkin’s lymphoma (NHL) of the tongue, particularly the anterior tongue, is extremely rare. NHL of the tongue accounts for 2.9-7.4% of extranodal NHLs of the head and neck region [5]. To the best of our knowledge, no cases of DLBCL arising on the inferior surface of the tongue have previously been reported in the English literature. The clinical features of primary NHL involving the tongue cannot be defined due to the very low incidence and unusual characteristics. Tongue localization of DLBCL in an AT patient has not previously been reported in so far as we know.
The optimal treatment for NHL in AT patients is controversial. Historically, treatment of malignancy in primary immunodeficient patients using conventional doses of chemotherapeutic and radiotherapeutic agents has met with limited success due to opportunistic infection and treatment-related toxicity. On the other hand, given the potential for severe side effects from chemotherapy and radiotherapy, AT patients require careful monitoring and should receive modified protocols of treatment [6].
High cure rates are possible in children with localized NHL using a variety of chemotherapeutic strategies. To reduce late sequelae, the duration and intensity of chemotherapy has been progressively reduced. The French-American-British Lymphoma Malins de Burkitt (FAB/LMB) 96 study reported long-term survival in children with resected stage I disease treatment using 2 courses of COPAD (cyclophosphamide, vincristine, prednisolone, and doxorubicin) [7]. In addition, St. Jude Children’s Research Hospital reported a study in which NHL in children with AT was treated using 2 courses of COPAD. To avoid severe complications in our AT patient after resection of stage I DLBCL, she was treated using a modified dosage of COPAD and retuximab.
After anticancer treatment, a variety of secondary hematological and solid malignancies have been described in AT patients [3]. Leung et al., reported data on second malignancies after treatment for childhood NHL, clearly showing that this subset of patients have around a 10.8-fold higher risk of developing secondary malignancies than the general population, mostly in the form of acute myeloid leukemia and breast cancer [8]. Determination of subsequent risk in AT patients diagnosed with one type of neoplasm revealed that approximately 25% of patients with solid Tumours subsequently developed NHL or leukemia. A very low risk of subsequent neoplasms existed when the first was lymphoid in origin [9].
Conclusion
Primary diffuse large B-cell lymphoma arising in the tongue is very rare, but ought to be considered during the differential diagnoses because of the characteristics of AT. In addition, AT shows a poor prognosis. We emphasize the need for careful and strict clinical and instrumental surveillance of the present case, with the aim of achieving diagnosis of malignancy as early as possible.
[1]. Taylor AM, Metcalfe JA, Thick J, Mak YF, Leukemia and lymphoma in ataxia telangiectasiaBlood 1996 87:423-38. [Google Scholar]
[2]. Rotman G, Shiloh Y, ATM: from gene to functionHum Mol Genet 1998 7:1555-63. [Google Scholar]
[3]. Makis A, Polychronopoulou S, Haidas S, Osteosarcoma as a second Tumour after treatment for primary non-Hodgkin’s lymphoma in a child with ataxia-telangiectasia: presentation of a case and review of possible pathogenetic mechanismsJ Paediatr Haematol Oncol 2004 26:444-46. [Google Scholar]
[4]. Zepater E, Bagan JV, Carbonell F, Basterra J, Malignant lymphoma of the head and neckOral Dis 2010 16:119-28. [Google Scholar]
[5]. Haidar Z, A review of non-Hodgkin’s lymphoma of the oral cavity 1950-1980J Oral Med 1986 41:197-200. [Google Scholar]
[6]. Sandoval C, Swift M, Treatment of lymphoid malignancies in patients with ataxia-telangiectasiaMed Paediatr Oncol 1998 31:491-97. [Google Scholar]
[7]. Gerrard M, Cairo MS, Weston C, Auperin A, Pinkerton R, Lambilliote A, Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international studyBr J Haematol 2008 141:840-47. [Google Scholar]
[8]. Leung W, Sandlund JT, Hudson MM, Zhou Y, Hancock ML, Zhu Y, Second malignancy after treatment of childhood non-Hodgkin lymphomaCancer 2001 92:1959-66. [Google Scholar]
[9]. Morrell D, Chase CL, Swift M, Cancers in 44 families with ataxia-telangiectasiaCancer Genet Cytogenet 1990 50:119-23. [Google Scholar]