Anaemia and cancer are inter-related. Many studies have demonstrated the dramatic adverse impact of anaemia upon loco-regional tumour control and survival and have revealed haemoglobin (Hb) levels as a powerful prognostic factor, a compelling evidence for the value of reversing anaemia and hence tumour hypoxia in head and neck cancer patients [8]. Irrespective of the cause, the existence of anaemia in cancer patients can impair the effectiveness of treatment and negatively impact the outcome [8].
Prognostic factors are the characteristics of patient which affect survival, treatment outcome and modify the therapeutic strategies. Patient related prognostic factors are weight loss of > 5%, an Eastern Co-operative Oncology Group (ECOG) performance status; Tumour-related prognostic factors are: site of the tumour, presence of tumour residue, the primary tumour (T) size, depth of invasion, Grade of tumour, presence or absence of vascular invasion, metastatic lymph nodes (N), and their size, number, and position (level), extracapsular tumour spread (ECS) [9]. Poor ECOG performance status is known to be an independent unfavourable prognostic factor [10].
The ECOG Scale of Performance Status (PS) quantifies the functional status of cancer patients, and is an important factor determining prognosis in a number of malignant conditions, describing the status of symptoms and functions with respect to ambulatory status and need for care [11].
Mucositis, inflammatory response of oral mucosa is one of the adverse effects of radiation which is quantified by Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [12].
Although anaemia is considered to be a contributor to intra-tumoural hypoxia and tumour resistance to ionizing radiation in cancer patients, most evidences are from developed nations, the exact role of anaemia in treatment outcome of HNSCC patients in developing world (where low haemoglobin levels are usual) needed further prospective investigation.
Materials and Methods
This is a non-interventional non-randomized single blinded study. All the investigations, interventions and treatment were being done as part of standard of care and not as part of the study. Our study was limited to collection of de-identified data in federal Health Insurance Portability and Accountability Act of 1996 (HIPPAA) compliant manner from patients attending our outpatient department consecutively, who met the eligibility criteria. Patients were stratified into Anaemic and non-Anaemic group based on the pre-RT Hb status of ≤ 12 g/dl. Stratification of the patients into two groups was done only during the analysis of the data at the end of our study.
Patient Evaluation: As a part of standard of care, the patients were evaluated with complete history, physical examination, clinical and endoscopic evaluation, blood and radiological investigations. Patients with pre-RT Hb status <10g/dl were given haematinic support and/or blood transfusion.
Staging work up and pre-radiotherapy investigations including the laboratory (haematology and biochemistry) as well as the radiological investigations (chest radiography, computed tomography of the site of the primary tumour and the neck) were as per American Joint Committee on Cancer (AJCC) Staging Criteria 7th Edition published in 2010 [13].
Patient population and eligibility criteria
Inclusion Criteria: 1) HNSCC of Stage III/IVA 2) Age of the patient ≥ 18 years and ≤ 70years. 4) ECOG Performance status 1 or 2. 3) Head and Neck Sites: oral cavity (OC), oropharynx (OP), hypopharynx (HP), larynx (LX) and nasopharynx (NP). 5) Patients undergoing concurrent chemoradiation. 6) At least 6 weeks of follow up after completion of treatment.
Exclusion Criteria: Patients with anyone of the following factors were excluded from the protocol.
1) Previous history of malignancy. 2) Previous history of radiation in head and neck site. 3) Patients with uncontrolled co-morbidity like Diabetes and Hypertension. 4) Patients having pre-morbid conditions precluding chemotherapy or radiotherapy. 5) Patients with other fatal and non-fatal co-morbid conditions that can affect the outcome of the treatment or the overall survival (OS) in general.
HNSCC from various sites such as oral cavity (OC), oropharynx (OP), hypopharynx (HP), larynx (LX) and nasopharynx (NP) were included in our study. The selection criteria enrolled patients who came for six weeks of follow-up, precluding any attrition in patient population after enrollment. The methodology included only the accounting for Haemoglobin level prior to radiation therapy. In addition to pre-RT Hb levels, patient variables such as age, gender, Tumour site, T stage, N stage, ECOG Performance status, grade of mucositis were analysed for correlation with ETR.
Radiation therapy: All eligible patients received concurrent chemotherapy. Radiation consisted of conventionally fractionated dose of 66Gy, 2 Gy per fraction, five fractions per week in 6.5 weeks. Radiation therapy technique was individualized for each patient. However, in general patients were treated in supine position with aquaplast mask for immobilization with shoulder retractor, treatment volume included the primary tumour site, involved lymph nodes and as well as all draining lymph nodes at risk.
Chemotherapy: The patients were treated concurrently with weekly Cisplatin 30 mg/m2 during radiation.
Statistical Analysis
All the analyses were done using Statistical Package for the Social Sciences (SPSS) version 17.0. All the categorical variables were expressed as either percentages or proportions. The continuous variables were expressed as mean ± standard deviation. Comparison of categorical variables was carried by using Chi-square test of Fisher’s exact test whichever is applicable. P-value <0.05 was considered statistical significance. Pre-RT Hb levels were a continuous variable in our study. In our study, mean pre-RT Hb level was 10.7 g/d (σ: 1.2). Response was compared below and above the value of 12 g/dl and also the mean value (Hb 10.7 g/dl).
ETR were categorized into Complete Response (CR), Partial Response (PR), Stable disease (SD) or Progressive disease (PD) as per Revised Response Evaluation Criteria in Solid Tumours (RECIST) guideline (version 1.1) [14].
Complete Response (CR) is defined as disappearance of all target lesions. Partial Response (PR) is defined as at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. Progressive Disease (PD) is defined as at least a 20% increase in the sum of diameters of target lesions taking as reference the smallest sum on study (this includes the baseline sum if that is the smallest on study). Stable Disease (SD) is defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameters while on study [14].
Computed Tomography (CT) scans of ≤5 mm slice thickness obtained during radiation treatment planning and during follow-up was used to measure the response. For easier analysis responses other than Complete Response (CR) were grouped into non-CR. ETR assessment at six weeks as measured by above criteria was the endpoint in our study. Different pre-RT Hb levels were analysed for correlation with ETR.
Results
Ninety one patients who met the eligibility criteria were enrolled and Patient characteristics (age at diagnosis, gender, and pre-RT Hb level), Tumour characteristics (tumour site, T stage, N stage and pathological grade) and Treatment Characteristics (radiotherapy technique – 3D Conformal Radiotherapy (3DCRT) with Intensity Modulated Radiation Therapy (IMRT), mucositis and treatment withholds were recorded and analysed [Table/Fig-1].
Characteristics of study population and treatment response
| Characteristics | n | CR | NO CR | p-value |
|---|
| Age in Years |
| ≤55 | 41 | 27 | 14 | 0.8 |
| >55 | 50 | 27 | 23 |
| Gender |
| Male | 71 | 40 | 31 | 0.31 |
| Female | 20 | 14 | 06 |
| Tstage |
| T3 | 27 | 21 | 6 | 0.138 |
| T4a/b | 64 | 33 | 31 |
| N stage |
| N1 | 21 | 09 | 12 | 0.215 |
| N2a | 09 | 07 | 02 |
| N2b | 26 | 16 | 07 |
| N2c | 35 | 19 | 16 |
| Pre-RT Hb in g/dl |
| <10.7 | 38 | 07 | 31 | < 0.0001 |
| ≥10.7 | 53 | 47 | 06 |
| Pre-RT Hb in g/dl |
| <12 | 67 | 34 | 33 | < 0.0001 |
| ≥12 | 24 | 20 | 4 |
| Mucositis |
| Grade 2 | 25 | 22 | 03 | 0.001 |
| Grade 3 | 66 | 32 | 34 |
| Treatment Breaks for ≥5 days |
| YES | 33 | 09 | 24 | <0.0001 |
| NO | 58 | 45 | 13 |
| ECOG Status |
| 1 | 43 | 35 | 08 | 0.0002 |
| 2 | 48 | 19 | 29 |
| Tumour Site |
| NP | 7 | 06 | 01 | 0.503 |
| OC | 18 | 10 | 08 |
| OP | 32 | 22 | 10 |
| HP | 23 | 13 | 10 |
| LX | 11 | 03 | 08 |
Patient characteristics: Our patient population was characterized by a mean age of 55.63 (range: 32-69). It was skewed towards male population with 71 males (78%) and 20 females (22%). It had a performance status of ECOG 1 in 43 (47%) and ECOG 2 in 48 (53%) patients. It was characterized by a Pre-RT Hb level of <10.7g/dl in 38 (42%) and ≥10.7 g/dl in 53 (58%) patients. Pre-RT Hb level was <12 g/dl in 67 (74%) and ≥12 in 24 (26%) patients.
Tumour characteristics: Our study population was characterized by tumours from various head and neck sites -nasopharynx 7 (8%), oral cavity 18 (20 %), oropharynx 32 (35%), hypopharynx 23 (25%) and larynx 11 (12%). The number of patients with T3 stage were 27 (30%) and T4 a/b stage were 64 (70%); with N1 stage 21 (23%) patients and N2a 9 (10%) patients, N2b 26 (28%) patients, N2c 35 (39%) patients.
Treatment Characteristics: All were treated by high precision radiation technique with 72 (79.12 %) being treated by 3DCRT and 19 (20.88 %) by IMRT. All had concurrent chemotherapy either weekly cisplatin or once in three weeks Cisplatin plus 5-FU regimen. The number of patients experiencing Grade 2 mucositis was 25 (27%) and Grade 3 mucositis was 66 (73%) patients. 58 (64%) patients completed treatment with breaks< 5 days and 33 (36%) with treatment breaks for ≥5 days.
Analysis: Age, Gender, Tumour site, T stage, N stage did not show statistical significance for ETR [Table/Fig-1]. The variables which showed statistical significance are presented below:
Performance Status and ETR: ECOG 1 group had better ETR compared to ECOG 2 group. Performance status showed statistical significance with outcome of response (p < 0.001).
Treatment Interruptions and ETR: Patients with treatment withholds less than five days showed better ETR compared to Treatment interruptions for five and more days showed statistical significance with outcome of response (p < 0.001).
Patients with grade 2 mucositis had a better ETR compared to grade 3 mucositis. Mucositis reactions showed statistical significance with outcome of response (p < 0.001).
Correlation of Pre-treatment Hb level and treatment response: We did our analysis based on WHO criteria for Anaemia [15], which defined the same as Hb level of less than 12 g/dl. Patients with Pre –RT Hb ≥ 12 g/dl better ETR compared to patients with Pre –RT Hb <12 g/dl. Pre-RT Hb ≥ 12 g/dl showed statistical significance with outcome of response (p < 0.001) [Table/Fig-2].
Pre-RT Hb more than & less than 12 g/dl and early response
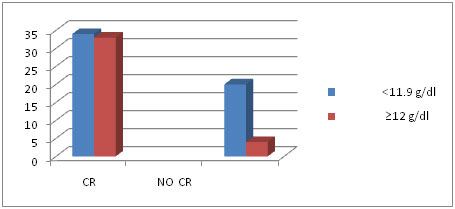
The Hb level of Indian population tends to be lower than international standards. The mean Hb level of our population was found to be 10.7 g/dl.
To present the more realistic scenario in India, we did our analysis based on this value too. Our results were as below:
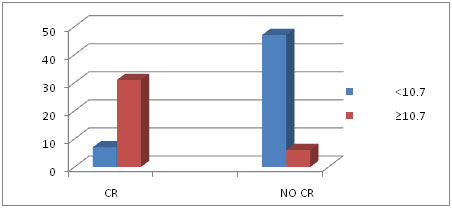
Patients with Pre–RT Hb ≥ 10.7 g/dl better ETR compared to patients with Pre –RT Hb <10.7 g/dl. Pre-RT haemoglobin ≥ 10.7 g/dl showed statistical significance with ETR (p< 0.001) [Table/Fig-3]. We also analysed various Pre-RT Hb levels serially. There was no statistical significance when the cohorts were divided based on Pre-RT Hb level ≤ 10 g/dl.
Pre-RT Haemoglobin more than & less than 10.7 g/dl and early response
We also analysed the correlation of the above prognostic factors showing significance to pre-RT Hb levels.
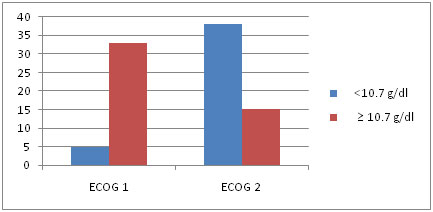
Relationship of ECOG performance status was analysed with pre-RT Hb. ECOG 1 group had better haemoglobin status compared to ECOG 2 group. ECOG performance status showed statistical significance with pre-RT Hb (p = 0.0002) [Table/Fig-4].
Performance status and Pre –RT Hb less than and more than 10.7g/dl
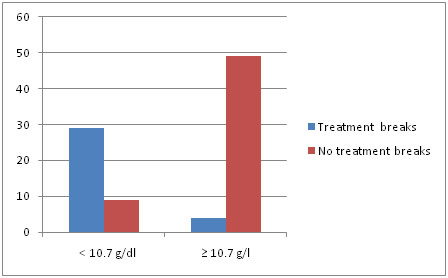
Patients with Pre –RT Hb ≥10.7 g/dl had fewer treatment interruptions compared to patients with Pre –RT Hb <10.7 g/dl. Treatment interruptions ≥ five days and pre RT Hb showed statistical significance (p = <0.0001) [Table/Fig-5].
Treatment withholds and Pre –RT Hb in g/dl
[Table/Fig-6] Patients with Pre –RT Hb ≥10.7 g/dl had lesser grade of mucositis compared to patients with Pre –RT Hb <10.7 g/dl. Mucositis reaction and pre-RT Hb showed statistical significance (p = <0.0001) [Table/Fig-7].
Mucositis reaction and Pre –RT Hb less than and more than10.7g/dl
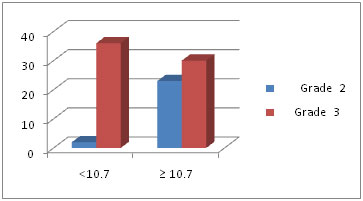
Pre-RT Hb with other factors
| Pre-RT Hb g/dl | <10.7 | ≥10.7 | p-value |
|---|
| Mucositis |
| Grade 2 | 02 | 23 | <0.0001 |
| Grade 3 | 36 | 30 |
| Treatment interruptions for ≥ 5 days |
| YES | 29 | 04 | <0.0001 |
| NO | 09 | 49 |
| ECOG Status |
| 1 | 05 | 38 | 0.002 |
| 2 | 33 | 15 |
Discussion
Only a few reports could be identified in the literature that prospectively evaluated prognostic factors of chemoradiation in HNSCC patients [16–22]. This is the first data on Indian population studying the prognostic significance of pre-RT Hb levels.
Danish Head and Neck Cancer (DAHANCA) Group Trial: DAHANCA 5 randomized HNSCC patients treated with the hypoxic radiosensitizer nimorazole or placebo, and in addition, patients with "low" pre-irradiation Hb values (females<13 g/dL; males<14.5 g/dL) were sub-randomized to plus or minus transfusion and found that, patients with high Hb levels had a significantly better probability of LRC, disease-specific survival (DSS) and overall survival (OS) compared to ‘low Hb no transfusion’ patients [23]. The univariate analysis (UVA) demonstrated prognostic significance of high Hb levels in HNSCC treated with radiotherapy; But in both UVA and multivariate analysis (MVA), transfusion prior to and during treatment did not improve the outcome in patients with low Hb values [24].
Further analysis by combining DAHANCA 5 and 7 to provide the statistical power for the analysis showed that patients with low Hb level had a significant reduced probability of LRC, DSS and OS. In the low Hb group, transfusion did not improve the outcome in LRC, DSS or OS. In MVA, there was no significant influence of transfusion or Hb level on endpoints [24].
Denis et al., reported on a cohort of Stage III or IV oropharynx cancer patients treated with radiotherapy alone or chemoradiation [16] that a pretreatment Hb level of <12.5 g/dL is a significant negative prognostic factor. A significant impact of the pretreatment Hb level on treatment outcome has also been reported for primary tumours of other sites such as esophageal cancer, lung cancer, and cervix cancer [20–22].
The range of Hb levels considered optimal for tumour oxygenation was reported to be 12–14 g/dL [22].The predictive value of the Hb may be explained by radioresistance associated with hypoxia. Tumour oxygenation can be affected by several factors such as adequacy of the blood supply, microcirculation, and oxygen-carrying capacity of the blood, might be represented by the Hb level [25]. This explanation is supported by our data, in particular by the fact that the higher Hb level was significantly associated with LRC.
Treatment Interruption: Another major finding of this study was the negative impact of radiotherapy interruptions more than five days resulting in poor LRC. These findings are in accordance with the available literature. The negative effect of interruptions during radiotherapy has also been described for various head and neck cancer sites. In a retrospective study on 1,102 patients, 1-day interruption was found to increase the risk of local failure by almost 5% [26]. Another study demonstrated a 3.5% decrease in control of the carcinoma larynx if the interruption lasted 5 days [27]. Few authors reported that the rate of loco-regional failure increases by 3.3% for each day of interruption in a retrospective series of 796 patients with nasopharyngeal carcinoma who received either continuous-course or split course radiotherapy [28]. Another retrospective review of 868 patients with head and neck cancer at various sites suggested that the time of the interruption during the series of radiotherapy has no impact on LRC [29].
Associated Findings: Third finding of this study was the patients with grade II mucositis had better treatment outcome compared to patients with grade III mucositis (p< 0.001). Patients with pre-RT Hb level of ≥10.7 g/dl had statistically significant lower incidence grade III mucositis compared to patients with patients with pre–RT Hb level of < 10.7 g/dl ( p<0.001) – signifying patients with higher pre- RT Hb level tend to suffer lesser grade of mucositis.
Fourth finding of this study was the patients with performance status ECOG 1 had better treatment outcome compared to patients with ECOG 2 (p< 0.001). Patients with pre –RT Hb level of ≥10.7 g/dl had statistically significant better performance status of ECOG 1 compared to patients with patients with pre –RT Hb level of < 10.7 g/dl ( p<0.001) – signifying patients with higher pre-RT Hb level tend to have better performance status.
The value of transfusion or poietic proteins in improving treatment outcome is controversial and needs further well designed studies. The idea of tumour biology being different and aggressive in anaemic patients is debatable and an area of potential research. It is important to assess pre-RT Hb to prognosticate and identify subgroup of patient population with poor outcome. It is necessary to avoid treatment interruptions to achieve the best possible treatment outcomes, which may require maximum supportive care during and after treatment and is in line with those, published prospective and retrospective data.
Conclusion
In our study the level of Hb having a significant effect on treatment outcome was at 10.7 g/dl. In addition to pretreatment Hb levels > 10.7 g/dL, we found that improved LRC in Stage III/IVA HNSCC is significantly associated with better performance status, lesser grade of mucositis and no interruptions or interruptions less than five days during radiotherapy.
However, definitive conclusions and recommendations need further expansion of our study for better statistical power and significance.