Introduction
Viral haemorrhagic fevers (VHFs) is a group of diseases caused by a variety of five discrete families of RNA viruses: Arenaviridae, Bunyaviridae, Flaviviridae, Filoviridae and Rhabdoviridae; ranging from non-severe illnesses like Lassa fever, Rift valley fever, Yellow fever and Dengue fever to more severe life threatening ones like Ebola virus disease, Marburg haemorrhagic fever and Crimean-Congo haemorrhagic fever. Severe forms are often characterized by extreme systemic manifestations such as widespread vascular damage resulting in extensive haemorrhage and multiple organ failure. VHFs are known to afflict regions widespread across the globe but mainly each illness is restricted to areas where the natural host of the virus resides. However, there have been instances when the virus has been exported from its natural habitat to distant locations.
Ebola haemorrhagic fever (Ebola HF), one of the several VHFs, is recognized worldwide as a severe, often deadly disease in humans and non-human primates (monkeys, chimpanzees and gorillas). It is caused by infection with virus of the genus Ebolavirus and family Filoviridae. Ebola virus was for the first time discovered to cause Ebola HF in 1976 in what is now known as the Democratic Republic of Congo (DRC) near the Ebola River. It is notorious for causing fatal outbreaks and epidemics in endemic regions of central, eastern and western Africa with lesser health threats beyond these areas. Five different subspecies of Ebolavirus are established, of which four are known to cause disease in humans. These, in their decreasing order of virulence and lethality, are Zaire virus (Zaire ebolavirus), Sudan virus (Sudan ebolavirus), Bundibugyo virus (Bundibugyo ebolavirus),Taï Forest virus (Taï Forest ebolavirus) and Reston virus (Reston ebolavirus) The earliest detected cases of Ebola virus outbreak were reported in Africa amongst the dead bodies and carcasses of chimpanzees and gorillas. The virus soon got transmitted from these animals to cause Ebola virus infection in humans.
History and Demographics
History of Ebola virus outbreaks can be traced to the first recorded outbreak in Democratic Republic of Congo (DRC), lying on the Ebola River. The first case of Ebola virus disease was identified on 26 August 1976, in Yambuku, a small rural village in Mongala District in northern DRC (then known as Zaire) [1]. Dr Peter Piot, along with his team members were the first ones to discover Ebola virus in the blood sample of a sick nurse working in Zaire in 1976. They later made key discoveries in establishing the transmission of this virus [2]. Ebola viruses are known for well-documented severe outbreaks of human haemorrhagic fever, with consequential case mortalities reaching as high as 85-95%. History and demographic details of various outbreaks of different species of Ebola virus are summarized in [Table/Fig-1,2,3,4,5] [3,4].
The current (2014) West Africa Ebola virus outbreak was significant as it was the largest and the most complex Ebola outbreak till date with an approximate total reported case count of 24,788 and reported death count of 10,251. It primarily involved four African countries—Guinea, Liberia, Sierra Leone and Nigeria [4]. The outbreak was formally designated as a public health emergency of international concern on August 8, 2014.
On July 23, 2014, 1,201 total suspected or confirmed cases (814 laboratory-confirmed) were reported in these countries, resulting in 672 deaths. Based on genetic analysis, the virus appeared to be mostly identical to Zaire Ebolavirus [5]. On August 20, 2014, the World Health Organization (WHO) and Centers for Disease Control (CDC) reported a total of 3,069 suspected cases and 1,552 deaths. On September 5, 2014, there was a reported increase in suspected cases and deaths acquiring a total of 3,967 and 2,105 (2,383 cases and 1,243 deaths being laboratory confirmed) respectively. The outbreak of Ebola Virus Disease (EVD) in Nigeria was declared over on October 19, 2014. A national EVD outbreak is considered to be over when 42 days (double the 21-day incubation period of the Ebola virus) has elapsed since the last patient in isolation becomes laboratory negative for EVD [6].
On December 2, 2014, there continued to be a rise in figures with a total of 17,256 suspected cases and 6,113 deaths (10,793 cases being laboratory confirmed) in Guinea, Liberia and Sierra Leone as reported by WHO and CDC [4]. On January 31, 2015, the count further rose to a total of 22,334 suspected cases and 8,921 deaths (13,373 cases being laboratory confirmed). Liberia was declared free of Ebola virus transmission on May 9, 2015.
Challenges during Ebola virus outbreaks
Ebola viruses are highly infectious in nature and were once the sole reason for contraction of this disease by a scientist while conducting an experiment in the laboratory. Because of their highly contagious characteristics, they have been responsible for some serious outbreaks in Africa. Moreover, several challenges that have prevented containment of the outbreak in Ebola hit areas include [7]:
• lack of knowledge and understanding amongst communities regarding epidemics,
• incapacity of prevailing health system in managing a disaster,
• lack of resources and experienced health care professionals,
• limited facility for timely and coordinated response amongst local, civil and government bodies,
• inadequate surveillance and monitoring,
• improper isolation and containment measures and
• Lack of in-country laboratory facilities for confirmation of causative agents.
Delay in identifying cases is yet another challenge attributed to difficulty in collection of clinical samples such as blood or serum from Ebola suspected cases and thereafter transporting them to the laboratory. Collection may be hindered because of non availability of sampling equipment, lack of adequately trained personnel, weak infrastructure in communication and transportation, sub standard laboratories and cultural restrictions while obtaining blood samples and other pre and post mortem invasive sampling [8]. Uganda is one country that has reported sporadic outbreaks of Ebola virus infection from 2001 till 2013 but post 2007, it has been able to successfully contain epidemics and drastically reduce mortality mainly owing to adoption and implementation of well planned and controlled strategies [7].
Types or Classification of Ebola Virus: Based on differences in genomes i.e. genetic sequencing, number and location of gene overlaps as well as immune reactions evoked by the virus; five different subtypes of Ebola virus genus have been recognized which include ZEBOV, SUDV, RESTV, BDBV and TAFV. These are characterized by varying virulence and pathogenicity [Table/Fig-6] [9].
Ebola virus pathogenesis
Ebola virus belongs to the Filovirus family, characterized by membrane enveloped filamentous particles in the shape of a shepherd’s crook or in the shape of a “U” or a “6” [Table/Fig-7]. It is comprised of three components namely viral envelope, matrix and nucleocapsid. The viral envelope is derived from cell membrane of the host during the budding process and it is here that the underlying viral encoded glycoproteins (GP) insert during transcriptional editing. Transmembrane glycoproteins, 150 – 170 kDA, are present on the inner lipid bilayer surface as 7-10nm long spikes and play a significant role in Ebola virus life cycle by mediating attachment, entry and fusion into target cells thus permitting replication and spread of virus. Other than the transmembrane GP, sGP, a soluble non structural secreted form of glycoprotein, 60-70 kDA, has less defined function. It is conjectured that sGP is not incorporated into the virus particle but is rather secreted from infected cells. Both gene products, sGP and transmembrane GP, possess discreet biochemical and biological properties which render distinctly separate roles in Ebola virus infection [10,11].
Matrix space between the virion envelope and central nucleocapsid houses viral matrix proteins, VP40 and VP24. VP40 mediates budding as well as viral particle release while VP24, a minor matrix protein, participates in nucleocapsid formation and assembly, also regulating viral transcription and replication. In the centre of the virion, a nucleocapsid is present which is composed of a series of viral proteins attached to a linear non segmented, single negative stranded RNA genome. Four nucleocapsid proteins: nucleoprotein NP, RNA polymerase L, polymerase cofactor VP 35 and transcription factor VP 30, are important for replication and transcription of viral genome [12].
Life cycle of Ebola virus begins with the binding and attachment of virion to specific surface receptors on target cell membrane mediated via glycoprotein subunits. The virion envelope subsequently fuses with the cellular membrane of host cells and the virus nucleocapsid is released into the cytosol resulting in viral uptake. The internalized nucleocapsid serves as a template for viral transcription and replication resulting in copying into full-length, positive-strand RNA antigenomes. These antigenomes serve as templates for transcription into negative-strand virus progeny genome copies. Newly synthesized structural proteins and genomes then self-assemble and accumulate under cell membrane, sites from where the virus is released. When budding off from the cell, virions gain envelope from the cellular membrane they bud from. These mature progeny particles infect other cells to repeat virion cycle; in this manner replicating at an extraordinarily high rate and exploiting cellular machinery, by overpowering protein synthesis apparatus of infected cells as well as host immune defenses [10-12]. The common host targets in Ebola virus infection are primary host targets which include monocytes, macrophages and dendritic cells; organ targets which include liver and adrenals and secondary targets which include fibroblasts and endothelial cells. Transmembrane GP compared to sGP forms a trimeric complex that binds the virus preferentially in the earlier stages of infection to cells of mononuclear phagocytic system i.e. monocytes and macrophages. Resultant exposure of these cells to viral particles causes cell damage resulting in release of cytokines (to be specific, TNF-α, IL-6, IL-8, macrophage chaemotactic protein (MCP)-1 and nitric oxide (NO) contributing towards fever and exaggerated inflammatory responses [13]. Early target attack combined with additional dendritic cell infection influences the innate and adaptive immune responses of the host resulting in rapid and extensive dissemination of the virus (even upto levels of 106.5 PFU/ml in blood). Widespread distribution of virus to various parts of the body is thus facilitated by blood flow containing free virus particles and infected monocytes, free virions and infected dendritic cells in lymphatic flow and cell to cell spread via cellular protrusions. Migration of infected monocytes into connective tissue infects fibroblasts promoting further spread to surrounding cells by cellular protrusions. A peculiar feature of fatal EVD cases is the minimal presence of inflammatory cells – neutrophils, lymphocytes and monocytes around viral infected cells contrary to non fatal cases where leukocyte concentration around infected cells may restrict viral dissemination [12]. As infection progresses, virus infects endothelial cells lining the inner surface of blood vessels resulting in vascular dysfunction and loss of vascular integrity precipitating haemorrhagic symptoms. Ultimately in advancing infections, infected macrophages, dendritic cells, endothelial cells and hepatocytes undergo non apoptotic cell death and necrosis
Secreted glycoprotein (sGP) does not participate in viral replication rather is secreted from infected cells. It forms a dimeric protein that interacts with neutrophils by mediating neutrophil binding either directly or indirectly through CD16b [10]. Neutrophil binding interferes with the signaling of neutrophils and aids the virus to evade host immune system by inhibiting early steps of neutrophil activation which ordinarily supports viral clearance. Further, neutrophils serve as carriers to disseminate virus throughout the entire body to places such as lymph nodes, liver, lungs, and spleen [13,14]. Thus, sGP alters immune response by inhibiting activation of neutrophils, while transmembrane GP may contribute to haemorrhagic fever symptoms by targeting virus to cells of the reticulo endothelial network and the lining of blood vessels [10].
Transmission
The exact natural reservoir host for Ebola virus still remains uncertain. On the basis of available evidence and nature of similar viruses, researchers believe that this virus is animal borne (zoonotic) and is totally dependent on its hosts for replication and overall survival [Table/Fig-8]. Birds, arthropods and plants are considered to be the possible reservoirs of Ebola virus but it is not yet conclusively established whether these are primary reservoirs or intermediate reservoirs getting infected from the primary reservoirs. Bats appear to be the most likely reservoir as there are hardly any clinical signs that can be found in them. In a recently conducted experiment that included 24 plant species and 19 vertebrates inoculated with Ebola virus, results showed bats to be infected, carrying and spreading the disease [15]. Specifically, the fruit bat species such as Epomops franqueti, Hypsignathus monstrosus, and Myonycteris torquata were found to carry the virus without showing any symptoms of the disease. Of the five known subtypes of Ebola virus, four types are known to dwell in an animal host which is native to Africa.
Spread of Ebola virus is known to occur through contact with infected animals or humans; most likely, through direct contact of broken skin or unprotected mucous membranes with virus-containing body fluids. Animal to human transmission takes place through contact with meat or body fluids of the infected animal. EVD shows no sexual predilection, but men and women differ with respect to the manner in which the direct exposure can occur. Men, owing to their nature of work exposure in forest and savanna regions, may be at an increased risk of acquiring primary infection while gathering “bush meat” (primate carcasses) for food, as well as from unknown vectors. Infection amongst humans can transmit through physical contact with infected skin or bodily fluids such as blood, feces or vomit. Ebola virus has additionally been detected in saliva, mucus, urine, semen and breast milk. Male survivors may be able to transmit the infection via semen for nearly two months. Routes of entry are open wounds, abrasions and cuts. The ‘hot virus’ as it so named may also get transmitted through conjunctival, nasal and oral exposure. In saliva, it has been found most frequently during the severe stage of illness.
Infected patients are not considered communicable prior to onset of symptoms i.e. during the incubation period of virus. Risk of transmission is also very low during the early onset of symptoms. However, there is an increase in communicability with each subsequent stage of illness with maximum chances of infection in later stages. Cases continue to remain communicable as long as blood and body fluid secretions contain the virus i.e. during convalescence period, before recovery and during post-mortem period. Ebola virus does not show any airborne transmission, but may inadvertently spread via droplets that are coughed or sneezed from a sick person. Subsequently, these droplets may enter the eyes, nose, or mouth of another person who is less than two meters away. Strategies should therefore be undertaken to reduce aerosol generation in aerosol generating medical procedures. Due to this potential characteristic of the virus, it has also been classified as a highly potential agent of bioterrorism.
Indirect transmission from surfaces or objects previously contaminated by blood or bodily fluids is a possibility but risk of transmission by this method is low. Medical workers are at a high risk of contracting the disease particularly when appropriate personal protective gear is not available or improperly used. Hospital acquired infections are likely when needles are reused and adequate containment measures are not practiced. It is therefore advised to properly handle and dispose contaminated medical equipment like syringes and needles.
Ebola Virus Disease (EVD) Symptoms
Ebola virus disease (EVD) is often deadly in humans and usually runs its course from 14 to 21 days. Its incubation period is typically 8 to 10 days, but can vary between 2 and 21 days. Infection initially presents with sudden nonspecific influenza-like symptoms characterized by fever, fatigue, headaches, joint, muscle and abdominal pain. Besides these, gastrointestinal symptoms like abdominal discomfort, nausea, vomiting, diarrhea and loss of appetite are also frequently seen. Other less common symptoms include: sore throat, chest pain, hiccups, dyspnoea and difficulty in swallowing. Presentations on skin may be in the form of a maculopapular rash in about 50% cases. In the later stages of illness, patients may suffer from profuse vomiting and diarrhea which unchecked could result in severe volume depletion, electrolyte imbalance and shock. Symptoms of EVD before progression into bleeding phase are very similar to those of malaria, dengue fever or other tropical fevers and hence warrant close monitoring throughout [16].
Bleeding phase typically sets in 5 to 7 days after the onset of first symptoms. In advancing stage of illness, the virus starts targeting microvascular endothelial cells resulting in loss of vascular integrity and leakage of blood [10]. Subsequent internal and subcutaneous bleeding manifests as haemorrhages under the skin seen as petechiae, purpura, ecchymoses and haemotomas particularly around needle injection or puncture sites. Bleeding from mucous membranes like gums, nose, gastrointestinal tract and vagina have also been reported. With further progression of infection, patients begin to display severe bleeding and coagulation abnormalities such as gastrointestinal bleeding (vomiting or coughing up blood and/or blood in the stool), rashes and haemotological irregularities like lymphopenia and neutrophilia. Liver damage associated with massive viremia leads to disseminated intravascular coagulopathy. Infected patients sometimes exhibit symptoms of circulatory system involvement, including impaired blood clotting [17]. On the whole, bleeding is generally indicative of poor prognosis as hypotensive shock resulting from blood loss due to diffuse bleeding often results in death. In the event of non recovery of an infected person, multiple organ failure contributing to death can be expected within 7 to 16 days (usually between days 8 and 9) after first symptoms [18]. Fatality rate is as high as 90% in EVD. Usually, if the patient is able to survive one week post onset of symptoms, recovery is rapid and complete.
Diagnosis
If EVD is suspected, thorough medical history including travel and work history as well as any exposure to wildlife in recent past is important to investigate. Laboratory tests that may be indicative include basic blood tests – Complete blood count (CBC) with differential, liver enzymes, bilirubin, creatinine levels, blood urea nitrogen (BUN) and pH. Diagnosis is confirmed by (1) isolating the virus and detecting its RNA and proteins or (2) detecting antibodies against the virus in a person’s blood. Isolating virus by tissue culture (to be performed only in high-containment laboratories), detecting viral RNA by polymerase chain reaction (PCR) and antigen detection by enzyme-linked immunosorbent assay (ELISA) are effective early and in those who have died from the disease. Serologic testing for demonstrating immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies against the virus is effective late in the disease and in those who recover [18].
Differential Diagnoses
Differential diagnosis includes VHFs such as Crimean-Congo haemorrhagic fever, Marburg haemorrhagic fever and others, malaria, typhoid, shigellosis, rickettsial diseases like typhus, cholera, gram- negative septicemia, leptospirosis, borreliosis, scrub typhus, plague, trypanosomiasis, visceral leishmaniasis, measles, haemorrhagic smallpox, and fulminant viral hepatitis. Non-infectious diseases such as haemolytic uremic syndrome, acute promyelocytic leukemia, clotting factor deficiencies/platelet disorders and thrombotic thrombocytopenic purpura. Hereditary haemorrhagic telangiectasia, warfarin poisoning and Kawasaki disease should also be considered in differential diagnosis [19].
Immune response in Ebola virus infection
In addition to precipitating exaggerated non protective systemic inflammatory responses and impairing vascular and coagulation systems, Ebola virus infection is also known to result in profound immune suppression giving little opportunity for development of natural immunity. Ebola virus acts both directly or indirectly to disable antigen-specific immune responses. Generally, lymphocytes in an infected individual continue to remain uninfected and are spared of viral replication, possibly because of lack of specific surface receptors essential for binding; but undergo apoptosis and subsequent clearing by surrounding macrophages [12]. Apoptosis of lymphocytes may be attributed to various intrinsic and extrinsic pathways, one of them being dysregulated dendritic cells and macrophages. Dendritic cells, which are primarily responsible for initiation of adaptive immune responses, are a major site where the virus replicates. Infected cells fail to undergo maturation and are unable to present antigens to naive lymphocytes. The later undergo “bystander” apoptosis, apparently induced by pro inflammatory cytokine mediators and/or loss of support signals from dendritic cells [20]. It is hypothesized that resultant depletion of lymphocytes due to extensive lymphocyte apoptosis arrests adaptive immunity and overwhelms viral pathogenesis. However, ensuing experiments lately have indicated that lymphocyte apoptosis is a byproduct of fatal infection and is not required for pathogenesis.
Passive transfer of serum or immunoglobulins from infected individuals to naïve individuals prior to exposure or immediately post exposure is known to confer protection in several viral fevers such as measles, rabies, polio, hepatitis A and Ebola infection. Protection offered by antibody transfer is mainly by (1) restricting viral replication and (2) some replication may be observed but it blunts the infection such that T cell and innate immunity are capable of resolving the infection. In animal models such as guinea pigs, immune serum containing high titers of neutralizing antibodies has known to confer protection in Ebola virus infections when administered prior to, rather than after, Ebola virus challenge [21]. Passive transfer of antibodies immediately post exposure in monkeys has shown to delay the onset of viremia and clinical signs but has not shown to alter their overall survival [22]. Human monoclonal neutralizing antibodies when used in guinea pigs showed good efficacy in post exposure prophylaxis by reducing viremia and increasing survival rate where as when using the same antibodies in macaques, they failed to protect the animals against lethal ZEBOV challenge [23,24]. Transfer of immune sera with antibodies specific to viral proteins other than glycoproteins has shown fewer efficacies.
In humans, EVD progress is so rapid that an infected individual may die even before antibodies are detected. However, in patients recovering from Ebola virus infection, antibody titers against Ebola virus GPs are readily demonstrable but their titers in serum may not be sufficient enough to provide any protection against infection or render neutralizing activity invitro, warranting use of immunoglobulin preparations that contain more concentrated neutralizing antibodies [23]. More recently, selected monoclonal antibodies isolated from the bone marrow of recovered patients have shown to effectively neutralize Ebola virus replication in vitro [10,25]. Collectively, these results indicate that there is a complex interaction between EBOV and host immune system. Immune responses are likely to vary depending on virus strain, type of animal model and type of vaccine. Also, antibodies alone may not confer complete protection in filovirus infection rather concomitant cellular immunity is integral to achieving protection. Both CD4+ and CD8+ T cell responses are critical in combating Ebola virus infection.
On comparing immune parameters, significant differences in immune responses have been noted in fatal and non fatal cases of Ebola infection. Baize et al., studied and described the immune responses of patients in two large Ebola virus outbreaks in Gabon in 1996 [26]. They found that despite similar viral antigen loads being present in survivors and nonsurvivors, immune responses were different in both groups. Fatal cases are generally associated with high levels of viremia, elevated releases of pro inflammatory cytokines like tumor necrosis factor alpha, interferon gamma, interleukins (IL-6, IL-8, IL-1) etc, widespread and profound lymphocyte apoptosis, suppressed B cell responses, decrease titers /absence of viral specific functional antibodies, depletion of CD8+ T cells and loss of peripheral NK cells [27]. Low levels of T cell cytokine RNA levels in peripheral blood are indicative of lack of development of adaptive immunity in individuals just preceding death. Survival on the other hand is dependent on initial or innate immune responses to infection. Non fatal cases usually demonstrate low levels of viremia, lack explosive cytokine releases, generate significantly higher levels of neutralizing antibodies and higher percentages of CD8+ T cells, more peripheral NK cells and sustained T cell cytokine RNA levels resulting in viral antigen clearance [27].
Vaccine development
Despite ongoing research in this field, none of the anti viral agents or vaccines for Ebola virus are currently approved for human use by the regulatory bodies. Currently available vaccines are still experimental and require full testing for safety and efficacy in humans.
Promising vaccines undergoing clinical trials are those that are derived from adenoviruses, Vesicular Stomatitis Indiana Virus (VSIV) or filovirus-like particles (VLPs) as these are well known to render protection in nonhuman primates [28,29]. In 2003, a vaccine using an adenoviral (ADV) vector carrying the Ebola spike protein was tested on crab-eating macaques. After twenty-eight days, when challenged with the virus, animals remained resistant [30]. A single shot blended vaccine based on attenuated recombinant Vesicular Stomatitis Virus (VSV) vector carrying equal parts of vaccine vectors – Zaire ebola virus glycoprotein, Sudan ebola virus glycoprotein and Marburg glycoprotein, when used in nonhuman primates was able to confer protection against three species of Ebola virus and Marburg virus thereafter opening clinical trials in humans [31]. Experimentally, recombinant Vesicular Stomatitis Indiana Virus (VSIV) expressing glycoprotein of EBOV or SUDV has been successfully used for postexposure prophylaxis in nonhuman primate models [32].
Other vaccine approaches are based on using DNA alone, DNA prime/adenovirus boost, and recombinant proteins. Gene-based vaccines owing to their safety and immunogenicity have proved increasingly attractive. Amongst the several gene-based vaccines, use of naked plasmid DNA to direct synthesis of immunogens within the host cells has shown successful application in animal models. Genetic immunization with plasmid DNA was developed in guinea pig model and proved the first successful vaccine for Ebola virus [33]. A three plasmid DNA vaccine encoding envelope glycoproteins from Zaire and Sudan virus as well as nucleoprotein has also demonstrated good tolerance and immunogenicity in phase 1 human trials [34]. Priming boosting immunization using combination of DNA immunization and boosting with adenoviral vectors encoding viral proteins has shown generation of cellular and humoral immunity in cynomolgus macaques. Dramatically enhanced immune responses, with a 30 fold or higher increase in antibody titer were reported. All vaccinated animals continued to be asymptomatic for 6 months with no detectable virus after the initial challenge [35].
Recently, successful development of a vaccine against Ebola in mice was reported. Unlike its predecessors, this could be freeze-dried and stored for longer periods to be used later in a possible outbreak. It is particularly desirable to have a vaccine based on single injection and one that can confer protection against multiple agents. Vaccines based on multiple doses may not be practical during outbreaks owing to lesser time available for deployment of vaccines and also increased costs. Immunization taking six months or longer impedes the counter-epidemic use of vaccines thus necessitating the development of a vaccine that has a quicker onset of effectiveness. In 2009, an experimental vaccine was made and tried by researchers at Canada’s national laboratory in Winnipeg to pre-emptively treat a German scientist who might have been infected with Ebola virus during a lab accident [36].
Treatment
Despite the fact that a number of experimental treatments for EVD are under study, no specific therapeutic agents are known to exist for treating or preventing Ebola virus infections [16]. Treatment for EVD is primarily based on supportive care therapy which includes balancing fluids and electrolytes to counter dehydration given by either oral or intravenous route, administering anticoagulants early in infection to prevent or control disseminated intravascular coagulation, administering procoagulants late in infection to control bleeding, maintaining oxygen status, managing nausea, fever and pain (avoid using aspirin or ibuprofen to decrease risk of bleeding), prescribing medications to treat secondary bacterial or fungal infections and minimizing invasive procedures [37]. Treatment instituted at an early stage increases chances of patient survival.
Therapeutic agents under consideration for treatment or prevention of EVD include the following:
• Estrogen receptor drugs used to treat infertility and breast cancer (clomiphene and toremifene)
• Favipiravir has proved useful in mouse models
• Nucleoside analogue inhibitors of S-adenosylhomocysteine hydrolase (SAH)
• Interferon beta
• Recombinant human interferon alfa-2
• Recombinant inhibitor of factor VIIa / tissue factor
• Activated protein
• Horse- or goat-derived immune globulins
• Human-derived convalescent immune globulin preparations
• Recombinant human monoclonal antibody against the envelope glycoprotein (GP) of Ebola virus
• DNA vaccines expressing either envelope glycoprotein or nucleocapsid protein genes of Ebola virus
In the United States, FDA’s animal efficacy rule can be used in combination with phase I clinical trials to exhibit reasonable safety for an experimental, unapproved drug and to obtain permission for treating people infected by Ebola with the drug under the expanded access program [38]. This rule exists because the normal path for testing the safety and efficacy of drugs is not possible for diseases caused by dangerous pathogens or toxins [38].
FDA allowed the following drugs to be used in people infected with Ebola under these programs during the 2014 outbreak.
• ZMapp (a monoclonal antibody vaccine)
• TKM-Ebola (RNA interference drug)
• Favipiravir (a drug approved in Japan for stockpiling against influenza pandemics)
Prognosis
EVD has a fatality rate ranging from 25%–90% in infected individuals. In the event of patient survival, quick and complete recovery may be expected. Complaints of muscle pains, joint pains, skin peeling, hair loss and testicle inflammation are often seen as long term complications in prolonged cases. Few ophthalmologic complications like sensitivity to light; excess tearing, iridocyclitis, iritis, choroiditis and blindness have also been noted.
Non availability of experimental treatments in the most affected regions during the 2014 outbreak spurred great controversy, with some calling for experimental drugs to be made more widely available in Africa on a humanitarian basis, and others warning that making unproven experimental drugs widely available would be unethical, especially in light of past experimentation conducted in developing countries by Western drug companies [39]. As a result of the controversy, the World Health Organization convened an expert meeting of bioethicists on 11 August 2014 to consider the implications of making the experimental treatment more widely available. The panel reached consensus that the circumstances warrant the use of unproven interventions with as yet unknown efficacy and adverse effects both for treatment and for prevention, and also said that deciding which treatments should be used and how to distribute them equitably were matters that needed further discussion [40].
Prevention and Control measures for health care workers (HCWs)
All possible measures should be undertaken to reduce exposure to virus and prevent transmission in a health care setting. The following means should be adopted [41]:
Assessment of risk: Risk assessment of patient is the primary step to determine the level of appropriate protective measures that need to be taken. Higher risk of exposure is present in cases that are convalescent, in later stages of infection, when patient is incapable of self care and procedures requiring contact with blood and fluids.
Source control: Place suspected or confirmed Ebola patients in complete isolation. Patients should be advised on proper respiratory hygiene, cough etiquettes and hand hygiene after toileting and vomiting. Only essential health care workers (HCW) entry with proper personal protective equipment (PPE) should be permitted in patient area. Trained personnel should be deputed for monitoring proper use, removal and disposal of PPE to avoid spread outside the patient’s room. AGMPs should be minimized and if at all needed should follow airborne precautions including use of respirators rather than masks. Strategies should be undertaken to prevent generation of aerosols.
Personal protection measures: HCWs should be properly trained and educated towards hazards of exposure to blood, bodily fluids and contaminated surfaces. Standard infection control measures including basic hand hygiene measures should be the minimum precautions that are exercised in a health care setting. Level of desired protection is guided by risk assessment and expected contact with potential pathogens and fluids. PPE should be donned properly in a clean place. Enhanced PPE is recommended for use in high risks of exposure. Workers should receive proper instructions, training and supervision in this regard. Removal of PPE is also equally important and should be done correctly to prevent contamination. To prevent self contamination, mucous membranes of eyes, nose and mouth should not be touched by hands.
Patient transportation: Transportation of patient in the hospital premises should be kept to a bare minimum and only when essential. Transportation should follow a direct route with entry restricted to other individuals. Before transportation, patient’s clothing and bedding should be changed. Transportation personnel should wear proper PPE. Receiving area for the patients should be minimally occupied and all workers here should also use PPE.
Sharps handling: Restrict the use of needles or sharps. Used needles need not be recapped and should be disposed off immediately in puncture proof containers. Caution should be exercised while disposing to avoid injury.
Precautions in laboratory: Sample collection and transportation should follow hospital laid down protocols. Samples should be sent to only high containment laboratories where lab personnel are also properly trained to handle the samples. Prior notification should be sent to the laboratory about the probable diagnosis.
Dedicated patient equipment: Non critical equipment should be dedicated for single patient use and preferably be disposable. Their disposal should be in a container labeled as ‘no touch bio hazardous’.
Equipment processing: Semi critical and critical items should be processed separately following routine sterilization protocols. Handle used linen, cutlery and equipment in a way that minimum exposure to skin and mucous membranes occurs and transfer of pathogens to other patients and surfaces is prevented. Soiled objects should be disposed in a no touch bio hazardous container.
Environment cleaning: Environment should be thoroughly cleaned and disinfected on a regular basis. Frequently touched surfaces should be disinfected more frequently with a broad spectrum virucidal disinfectant. Like all other personnel, cleaning staff should also get education and hands on training courses and adopt proper PPE protocols.
Handling deceased patients: Proper PPE should be worn when handling dead bodies as even at this stage contamination is a possibility. Devices like catheters and endotracheal tubes should not be removed but wrapped along with the body in high quality plastic shroud. Double protection by wrapping in leak proof body bag is provided. Its exterior surface is rubbed with a surface disinfectant. Body bag should not be reopened.
Patient education: Patients should be educated towards the need for prevention, preventive measures undertaken, duration for prevention and a special emphasis on hand and respiratory hygiene.
Visitor education and management: Visitors should also be educated, monitored and screened for EVD prior to entering the hospital. They should not be permitted into the patients room rather should be restricted to the patient care area or waiting room. Visitors should also wear PPE in accordance with the hospital guidelines.
Table showing history of Zaire Ebola Virus Outbreaks
| Year | Location | Reported Cases, No. | Deaths, No. (%) |
|---|
| 1976 | Zaire | 318 | 280 (88) |
| 1977 | Zaire | 1 | 1 (100) |
| 1994 | Gabon | 52 | 31 (60) |
| 1995 | DRC | 315 | 250 (81) |
| Jan 1996 to Apr 1996 | Gabon | 37 | 21 (57) |
| Jul 1996 to Jan 1997 | Gabon | 60 | 45 (74) |
| 1996 | South Africa (acquired in Gabon) | 1 | 1 (100) |
| Oct 2001 to Mar 2002 | Gabon | 65 | 53 (82) |
| Oct 2001 to Mar 2002 | DRC | 59 | 44 (75) |
| Dec 2002 to Apr 2003 | DRC | 143 | 128 (89) |
| Nov 2003 to Dec 2004 | DRC | 35 | 29 (83) |
| 2007 | DRC | 264 | 187 (71) |
| Dec 2008 to Feb 2009 | DRC | 32 | 15 (47) |
| July 2012 | Uganda | 24 | 17 (71) |
| Nov 2012 | DRC | 77 | 36 (46) |
| Dec 2012 | Uganda | 7 | 4 (57) |
| Total | | 1490 | 1141 (76.6) |
Data from Centers for Disease Control and Prevention and World Health Organization
Table showing history of Sudan Ebola Virus Outbreaks
| Year | Location | Reported Cases, No. | Deaths, No. (%) |
|---|
| 1976 | Sudan | 284 | 151 (53) |
| 1976 | England* | 1 | 0 (0) |
| 1979 | Sudan | 34 | 22 (65) |
| 2000-2001 | Uganda | 425 | 224 (53) |
| 2004 | Sudan | 17 | 7 (41) |
| 2011 | Sudan | 1 | 1 (100) |
| Total | | 762 | 405 (53) |
Data from Centers for Disease Control and Prevention and World Health Organization
*Occurred after laboratory accident.
Table showing history of Tai Forest (Ivory Coast, Côte-d’Ivoire) Ebola virus outbreaks (no deaths reported)
| Year | Location | Reported Cases, No |
|---|
| 1994 | Côte-d’Ivoire | 1 |
| Total | | 1 |
Data from Centers for Disease Control and Prevention and World Health Organization.
Table showing history of Bundibugyo Ebola Virus Outbreak
| Year | Location | Reported Cases, No. | Deaths, No. (%) |
|---|
| Dec 2007 to Jan 2008 | Uganda | 149 | 37 (25) |
| June to Nov 2012 | Democratic Republic of the Congo | 36 | 13 (36.1) |
| Total | | 185 | 50 (27) |
Data from Centers for Disease Control and Prevention and World Health Organization.
Table showing history of Reston Ebola Virus Outbreaks (no deaths reported)
| Year | Location | Proven* Cases Reported, No |
|---|
| 1989 | Virginia, Texas, Pennsylvania | 0 |
| 1990 | Virginia and Texas | 4 |
| 1989-1990 | Philippines | 3 |
| 1992 | Italy | 0 |
| 1990 | Alice, TX | 0 |
| 1996 | Philippines | 0 |
| Nov 2008 | Philippines† | 6 |
| Total | | 13 |
Data from Centers for Disease Control and Prevention and World Health Organization
*Humans with serologic evidence of infection but without clinical disease
†Associated with pig farming
Types and brief description of five different recognized sub species of Ebola Virus [9]
| 1. Zaire ebolavirus (ZEBOV), now known as Ebola (EBOV) virus | Ebola virus outbreaks took place for the first time in Yambuku, Zaire in the year 1976. The virus causing such outbreaks has been named as Zaire Ebola virus. It is the most dangerous species of Ebola virus that has claimed to have the largest number of Ebola virus victims leading to highest number of Ebola virus deaths. Mortality rate is 80-90%. Its symptoms include a chilly feeling accompanied with high fever which shares its similarity with the symptoms of malaria. |
| 2. Sudan ebolavirus (SEBOV) | This is a second kind of Ebola virus species believed to have originated in Nzara, Sudan in 1976. The first cases of Sudan Ebola virus can be traced to the cotton factory workers of Sudan who were exposed to the same. The outbreak of Sudan Ebola virus was simultaneous with the outbreak of Zaire Ebola virus. The disease also broke out in 1979, 2000 and 2004, most recently. The agent of transmission for Sudan Ebola virus is still unknown but it has an average fatality rate of 41- 65%. |
| 3. Bundibugyo ebolavirus (BDBV) | Outbreak of Ebola virus disease in the Bundibugyo district of Uganda in 2007 and 2008 led to the detection of a species of Ebola virus that was hitherto unknown. This Ebola virus was named after its place of outbreak and there were at least 100 or more Ebola virus victims. It has a reported fatality rate of 30%. |
| 4. Ivory Coast ebolavirus (CIEBOV) / Taï Forest ebolavirus (TAFV) | The origin of Ivory Coast Ebola virus can be traced to Tai forests of the Côte d’Ivoire in Africa, where a female ethnologist conducting necropsy on a dead infected chimpanzee got herself accidentally infected. The initial outbreak was among the wild African chimpanzees in 1994. The next outbreak of this virus took place in 1995 in south of DRC. Its infection has reported only one non fatal case. |
| 5. Reston ebolavirus (REBOV) | It was discovered in 1989 in Reston, Virginia when infected monkeys were imported into Reston from Philippines. It has shown to cause disease in nonhuman primates such as monkeys, but is non pathogenic in humans. Deaths have not been reported with this virus as infected individuals have shown sero conversion and survival. Ebola Reston virus outbreaks have thereafter occurred in Texas and Italy. Interestingly, Ebola Reston virus in pigs has been detected very recently in its outbreak in Philippines. |
Ultrastructure of Ebola virus showing its three components: viral envelope, matrix and nucleocapsid
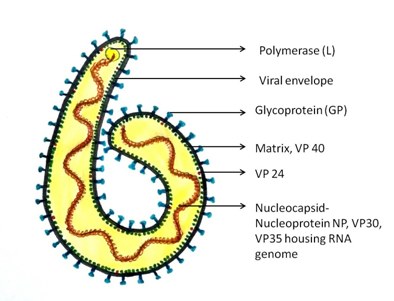
Figure showing transmission of Ebola virus. Contact with infected bats, duikers and non human primates can spread infection from animals to humans, following which human-human transmission can occur

Conclusion
Ebola virus disease (EVD), a filovirus haemorrhagic fever, is often a devastating disease in humans as it involves the body’s vascular system resulting in significant internal bleeding and multiorgan system involvement. Death due to EVD is mostly attributed to the resultant shock that sets in. Most Ebola outbreaks have been reported in equatorial Africa and have remained a cause of great concern and fear across the world owing to their high lethality, as high as 90% and risk of spread due to transport exportation. Ebola virus is highly contagious and because of its potential aerosol and droplet transmissibility, it is included in the ‘category A’ of bio terrorism agents. Till date, no effective prophylaxis, anti viral treatment, or vaccination for this fatal disease is available; therefore, increasing awareness of risk factors for Ebola infection and understanding protective measures which individuals can take is perhaps one of the most effective ways to reduce human infection and death.
Data from Centers for Disease Control and Prevention and World Health Organization
Data from Centers for Disease Control and Prevention and World Health Organization*Occurred after laboratory accident.
Data from Centers for Disease Control and Prevention and World Health Organization.
Data from Centers for Disease Control and Prevention and World Health Organization.
Data from Centers for Disease Control and Prevention and World Health Organization*Humans with serologic evidence of infection but without clinical disease†Associated with pig farming
[1]. A Augustine, Ebola research paper-academia.edu[Internet]. 2014 August [updated 2015 March 15; cited 2015 March 30]. Available from: http://www.academia.edu/8074025/EBOLA_RESEARCH_PAPER [Google Scholar]
[2]. S Pattyn, GV Groen, W Jacob, P Piot, G Courteille, Isolation of Marburg-like virus from a case of haemorrhagic fever in ZaireThe Lancet 1977 309(8011):573-74. [Google Scholar]
[3]. Outbreaks chronology: Ebola virus disease [Internet].Centres for Disease Control and Prevention [updated 2015 May 14; cited 2015 May 15]. Available from: http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html [Google Scholar]
[4]. 2014 Ebola Outbreak in West Africa - Case Counts [Internet]. Centres for Disease Control and Prevention 2015 March 24 [updated 2015 March 26; cited 2015 March 30] Available from: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html [Google Scholar]
[5]. JW King, Ebola virus infection.[Internet] http://misc.medscape.com/pi/iphone/medscapeapp/html/A216288-business.html [Google Scholar]
[6]. CDC emergency partners update 2014 Ebola response. [Internet] 2014 December 17 [cited 2015 March 30] http://content.govdelivery.com/accounts/USCDC/bulletins/e3b067 [Google Scholar]
[7]. AK Mbonye, JF Wamala, M Nanyunja, A Opio, I Makumbi, JR Aceng, Ebola viral haemorrhagic disease outbreak in West Africa- lessons from UgandaAfrican Health Sciences 2014 14(3):495-501.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4209631/pdf/AFHS1403-0495.pdf [Google Scholar]
[8]. P Formenty, EM Leroy, A Epelboin, F Libama, M Lenzi, H Sudeck, Detection of Ebola virus in oral fluid specimens during outbreaks of Ebola virus haemorrhagic fever in the Republic of CongoClinical Infectious Diseases 2006 42:1521-26. [Google Scholar]
[9]. C Ezeikpe, 10 most incurable diseases [Internet] 2014 January 8 [cited 2015 March 20]. http://yourlifeisgold.blogspot.in/2014_01_01_archive.html [Google Scholar]
[10]. N Sullivan, ZY Yang, GJ Nabel, Ebola virus pathogenesis: implications for vaccines and therapiesJournal of Virology 2003 77(18):9733-7.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC224575/ [Google Scholar]
[11]. A Sanchez, SG Trappier, BWJ Mahy, CJ Peters, ST Nichol, The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editingProc Natl Acad Sci USA 1996 93:3602-07. [Google Scholar]
[12]. J Olejnik, E Ryabchikova, RB Corley, E Muhlberberger, Intracellular events and cell fate in filovirus infectionViruses 2011 3:1501-31. [Google Scholar]
[13]. Ebola virus disease – Wikipedia, the free encyclopedia [cited 2015 March 30] http://en.wikipedia.org/wiki/Ebola_virus_disease [Google Scholar]
[14]. H Feldmann, H Bugany, F Mahner, HD Klenk, D Drenckhahn, HJ Schnittler, Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages J Virol 1996 70:2208-14. [Google Scholar]
[15]. R Swanepoel, PA Leman, FJ Burt, NA Zachariades, LE Braack, TG Ksiazek, Experimental inoculation of plants and animals with Ebola virusEmerging Infectious Diseases 1996 2(4):321-25. [Google Scholar]
[16]. AA Ansari, Clinical features and pathobiology of Ebolavirus infectionJ of autoimmunity 2014 55:1-9. [Google Scholar]
[17]. SP Fisher-Hoch, GS Platt, GH Neild, T Southee, A Baskerville, RT Raymond, Pathophysiology of shock and haemorrhage in a fulminating viral infection (Ebola)J Infect Dis 1985 152(5):887-94. [Google Scholar]
[18]. DIH Simpson, Marburg and Ebola virus infections: a guide for their diagnosis, management, and control[Internet] [Cited 2014 Oct 29].WHO Offset Publication. 1977; 36:10. Available from: http://www.whqlibdoc.who.int/offset/WHO_OFFSET_36.pdf [Google Scholar]
[19]. JH Gear, Clinical aspects of African viral haemorrhagic feversReviews of infectious diseases 1989 11(Suppl 4):S777-82. [Google Scholar]
[20]. A Baskerville, SP Fisher-Hoch, GH Neild, AB Dowsett, Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infectionJ Pathol 1985 147(3):199-209. [Google Scholar]
[21]. PB Jahrling, TW Geisbert, JB Geisbert, JR Swearengen, M Bray, NK Jaax, Evaluation of immune globulin and recombinant interferon-α2b for treatment of experimental Ebola virus infectionsJ Infect Dis 1999 179(Suppl 1):S224-S34. [Google Scholar]
[22]. PB Jahrling, J Geisbert, JR Swearengen, GP Jaax, T Lewis, JW Huggins, Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horsesArch Virol Suppl 1996 11:135-140. [Google Scholar]
[23]. PWHI Parren, TW Geisbert, T Maruyama, PB Jahrling, DR Burton, Pre and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibodyJ virol 2002 76:6408-12. [Google Scholar]
[24]. WB Oswald, TW Geisbert, KJ Davis, JB Geisbert, NJ Sullivan, PB Jahrling, Neutralizing antibody fails to impact the course of Ebola virus infection in monkeysPLoS Pathog 2007 3(1):e9 [Google Scholar]
[25]. CC Maruyama, T Rodriguez, PB Jahrling, A Sanchez, AS Khan, ST Nichol, Ebola virus can be effectively neutralized by antibody produced in natural human infectionJ Virol 1999 73:6024-30. [Google Scholar]
[26]. S Baize, EM Leroy, MC Georges-Courbot, M Capron, J Lansoud-Soukate, P Debre, Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patientsNat Med 1999 5:423-26. [Google Scholar]
[27]. SB Bradfute, S Bavari, Correlates of immunity to Filovirus infectionViruses 2011 3:982-1000. [Google Scholar]
[28]. Ebola/Marburg Vaccine Development (Press release). 2008 September 15. National Institute of Allergy and Infectious Diseases. [Google Scholar]
[29]. KM Daddario-diCaprio, TW Geisbert, U Stroher, JB Geisbert, A Grolla, EA Fritz, Postexposure protection against Marburg haemorrhagic fever with recombinant Vesicular stomatitis virus vectors in non-human primates: an efficacy assessmentLancet 2006 367:1399-404. [Google Scholar]
[30]. NJ Sullivan, TW Geisbert, JB Geisbert, L Xu, ZY Yang, M Roederer, Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primatesNature 2003 424(6949):681-84. [Google Scholar]
[31]. TW Geisbert, JB Geisbert, A Leung, KM Daddario-Di Caprio, E Hensley, Single-Injection Vaccine Protects Nonhuman Primates against Infection with Marburg Virus and Three Species of Ebola VirusJ Virol 2009 83(14):7296-304. [Google Scholar]
[32]. TW Geisbert, KM Daddario-DiCaprio, KJ Williams, JB Geisbert, A Leung, F Feldmann, Recombinant Vesicular Stomatitis Virus Vector Mediates Postexposure Protection against Sudan Ebola Haemorrhagic Fever in Nonhuman PrimatesJ Virol 2008 82(11):5664-68. [Google Scholar]
[33]. L Xu, A Sanchez, Z Yang, SR Zaki, EG Nabel, ST Nichol, GJ Nabel, Immunization for Ebola virus infectionNat Med 1998 4:37-42. [Google Scholar]
[34]. JE Martin, NJ Sullivan, ME Enama, IJ Gordon, M Roederer, RA Koup, VRC 204 Study Team. A DNA vaccine for Ebola virus is safe and immunogenic in a Phase 1 clinical trialClinical and vaccine immunology 2006 13(11):1267-77. [Google Scholar]
[35]. NJ Sullivan, A Sanchez, PE Rollin, ZY Yang, GJ Nabel, Development of a preventive vaccine for Ebola virus infection in primatesNature 2000 408:605-09. [Google Scholar]
[36]. Canadian made Ebola vaccine used after German lab accident. The Canadian Press. CBC News (Canadian Broadcasting Corporation). 2009 March 20. Available at: http://www.cbc.ca/news/technology/canadian-made-ebolavaccine-used-after-german-lab-accident-1.827949 [Google Scholar]
[37]. JH Choi, MA Croyle, Emerging targets and novel approaches to Ebola virus prophylaxis and treatmentBio Drugs 2013 27(6):565-83. [Google Scholar]
[38]. Moor on Ebola vaccine/ treatment [Internet] 2014 October 16. Available from: http://theamberinstitute.com/moor-on-ebola-vaccinetreatment/ [Google Scholar]
[39]. Ebola virus epidemic in west Africa. 2014 October 02 [cited 2015 March 20]. Available from: http://2014ebolaoutbreak.com/2014-ebola-outbreak-westafrica/ [Google Scholar]
[40]. N Nayyar, Ebola: complete information on sources, symptoms, diagnosis and treatment. [Internet] 2014 August 18 [cited 2015 March 20]. Available from http://www.womenfitness.net/ebola_sources.htm [Google Scholar]
[41]. Infection Prevention and Control Expert Working Group. Advice on Infection Prevention and Control Measures for Ebola Virus Disease in Healthcare Setting. [Internet]2015 February 23 [cited 2015 March 30]. Available at http://www.phac-aspc.gc.ca/id-mi/vhf-fvh/ebola-ipc-pci-eng.php [Google Scholar]