Oral cancer is the sixth most common malignancy and is a major cause for morbidity and mortality [1]. Globally about 5 lacs new oral and pharyngeal cancers are diagnosed annually. It is already established that oral carcinomas constitute 40-47% of total carcinomas affecting the body. Cancer has a multifactorial aetiology and is a multistep process involving initiation, promotion and tumour progression. It arises from a series of genetic alterations which leads to cellular proliferation and differentiation [2]. Cellular proliferation is one of the important indicator for the biologic aggressiveness of a malignant lesion.
Many biologic markers provide information on differentiation, proliferation and prognosis. Among this proliferating cell nuclear antigen (PCNA) is an acidic nuclear protein that fluctuates during cell cycle and increases in the late G1 to S phases and is well correlated with cell proliferation [3].
The aim of the study was to find the expression of PCNA by immunohistochemistry and to use it as a proliferation marker in normal, oral – premalignancy and squamous cell carcinoma.
Materials and Methods
The present study was carried out in the Department of Oral Pathology, VMSDC, Salem on the archival blocks comprising of 30 histopathologically proven cases of different grades of oral squamous cell carcinoma, 10 blocks of leukoplakia and 10 blocks of normal mucosa.
The study samples were divided into five groups. Group I consisted of blocks of normal mucosa of oral cavity which served as control, Group II consisted of blocks of oral pre malignancy, Group III consisted of blocks of well differentiated oral squamous cell carcinoma, Group IV consisted of blocks of moderately differentiated oral squamous cell carcinoma, Group V consisted of blocks of poorly differentiated oral squamous cell carcinoma.
Inclusion Criteria
Archival blocks of patients between the ages of 40-60 years.
Blocks of histopathologically diagnosed cases of dysplasia, oral squamous cell carcinoma and normal oral mucosa.
Exclusion Criteria
Archival blocks belonging to patients in other age groups.
Methodology
Each block was subjected to two sets of sections of 4 microns thickness. One set of sections were stained with routine haematoxylin and eosin stain for reconfirmation. After verification, the other set of sections were subjected to immunohistochemical evaluation using monoclonal PCNA antibody PC10 (Dako lab). The technique was based on the labeled streptavidin- biotin method. A known positive and negative control was also taken along with each batch. A previously diagnosed case of poorly differentiated oral squamous cell carcinoma stained with PCNA served as positive control where as a tissue from the present study without staining using the primary antibody was taken as negative control. The sections stained by PCNA were examined under light microscope. Highly mitotic cells took up positive PCNA stain and it was seen as light brown granular stain of the nucleus. Each case was analysed for PCNA positive cells and zones with positive staining only were used for counting. Areas of inflammation and stromal cells were excluded from counting. A minimum of 1000 tumour cells from 3 microscopic fields were counted using 40X magnification manually. Eyepiece mounted with counting grids was used for counting. A single examiner counted the positively stained cells in the same field, three times to eliminate intra examiner variability and an average value was taken.
Statistical Analysis
One-way ANOVA test, Fischer’s exact test (F-test) / variance-ratio test and Student’s t-test were carried out and p-value was obtained.
Results
Specimens from group 1showed an average count of 160.60, with a variance of 1741.60 and S.D of 41.732, Group 2 showed an average count of 229.60, with a variance of 2060.267 and S.D of 49.39, group 3 showed an average count of 457.70, with a variance of 923.788 and S.D of 30.3939, Group 4 showed an average of 532.20, with an variance of 6827.51 and S.D of 82.628, Group 5 showed an average of 643.40, with a variance of 5119.37 and S.D of 71.549 [Table/Fig-1].
PCNA Index of Different Groups
| Groups | Count | Average PCNA index | Variance | SD | p-value |
|---|
| Group I | 10 | 160.6 | 1741.6 | 41.73248 | 0.001 |
| Group II | 10 | 229.6 | 2060.267 | 49.39016 |
| Group III | 10 | 457.7 | 923.7889 | 30.3939 |
| Group IV | 10 | 532.2 | 6827.511 | 82.62875 |
| Group V | 10 | 643.4 | 5119.378 | 71.54983 |
p-value <0.05 is considered significant
p=0.001
Group I - Normal
Group II - Leukoplakia
Group III - Well differentiated Squamous cell carcinoma
Group IV - Moderately differentiated Squamous cell carcinoma
Group V - Poorly differentiated Squamous cell carcinoma
Based on the ANOVA test the sum of squares (SS) between groups was 1662878 with 4 degrees of freedom (df), with a mean of squares (MS) being 415719.4. SS within the group was 150052.9, with 45 df and MS being 3334.5. Evaluating all these values provide a significant F and p-values as 124.6719 and 0.001 respectively [Table/Fig-2]. One-way ANOVA test was performed and probability value of 0.001 was determined.
Comparison Of PCNA Index between And Within Groups
| Source of Variation | SS | df | MS | F | p-value |
|---|
| Between Groups | 1662878 | 4 | 415719.4 | | |
| Within Groups | 150052.9 | 45 | 3334.509 | 124.6719 | 0.001 |
| Total | 1812931 | 49 | | | |
p-value <0.05 is considered significant
p=0.001
Discussion
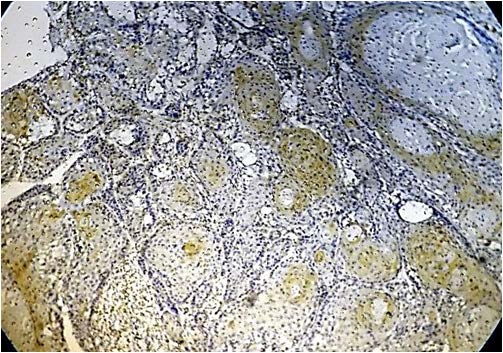
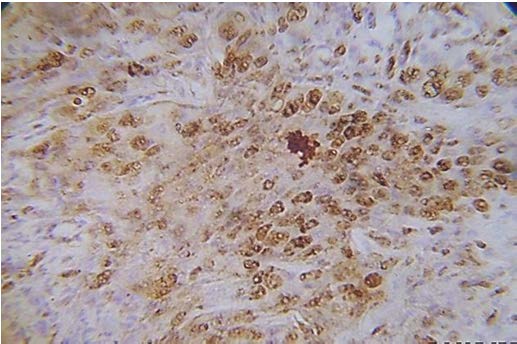
Present study was performed on archival tissues diagnosed as oral premalignancy and oral squamous cell carcinoma with differing histological grades. Identifying the diverse cell populations and analysing their rate of proliferation may help in predicting the prognosis of a particular patient [4]. In our study, the normal mucosal epithelium showed PCNA stained cells only along the basal cell layer [Table/Fig-3]. Dysplastic epithelium showed PCNA positive cells in basal and supra basal cells [Table/Fig-4]. Different grades of Oral squamous cell carcinoma showed a staining pattern from top to bottom of the epithelium and intense staining of the malignant cells inside the connective tissue [Table/Fig-5,6,7]. All the photomicrographs were taken in 10X magnification. It was observed that the intensity of staining and the number of positively stained cells increased gradually from Group I to Group V. [Table/Fig-8] shows anaplastic and hyperchromatic cells of poorly differentiated oral squamous cell carcinoma under 40X magnification.
PCNA stained cells in normal epithelium (10X)
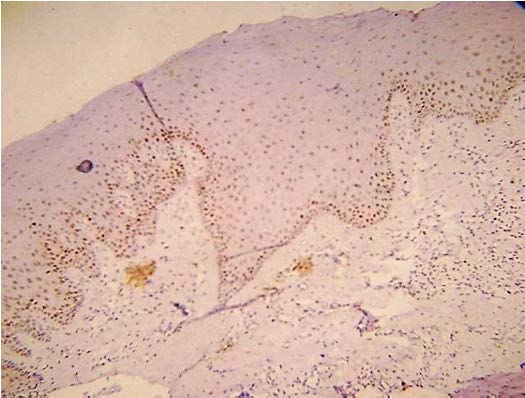
PCNA stained cells in dysplastic epithelium (10X)
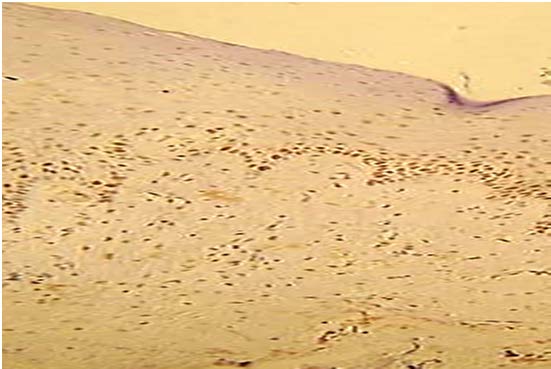
PCNA stained cells in poorly differentiated squamous cell carcinoma (10X)
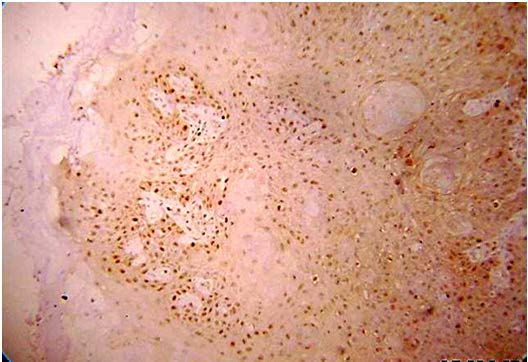
PCNA stained epithelium in Well Differentiated Squamous cell carcinoma (10X)
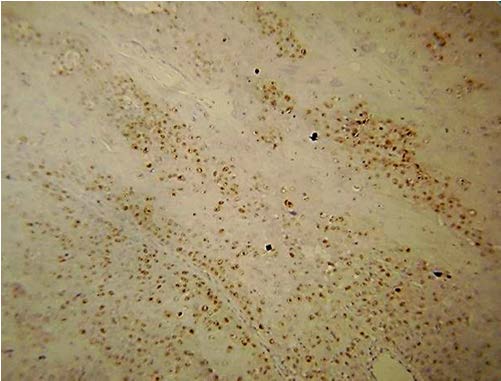
PCNA stained cells in moderately differentiated squamous cell carcinoma (10X)
Highly positive PCNA cells in Poorly differentiated Oral squamous cell carcinoma (40 X)
PCNA, is a 36 kD non-histone protein, the auxiliary factor of DNA polymerase delta [5]. Proliferating cell nuclear antigen – gene was isolated in humans which is located at chromosome 20p12. The synthesis of this protein correlates with the proliferative state of the cell as it is expressed in the nuclei of cells during the DNA synthesis [6].
PCNA is present in variable amounts in normal proliferating and transformed cells from a variety of vertebrates. Studies of PCNA have shown that its nuclear distribution changes dramatically through the S-phase and that it is tightly associated with DNA replication sites. The synthesis of PCNA is regulated during the cell cycle, increasing during the DNA replication period [7].
Tsuji et al., identified tumour proliferation as an important predictor of biologic behaviour in various malignant tumours. PCNA is one of the proliferation proteins and the antibodies to this protein would help to assess the tumour proliferation activity of oral lesions [8].
Rüdiger G Steinbeck et al., analysed varying degrees of atypia in squamous cell carcinomas located in the oral cavity, by means of PCNA immunoreactivity. Focal immunoreactivity of PCNA was noticed in highly differentiated squamous cell carcinoma and strongly elevated proliferation, aneuploidy was seen in the entire tumour mass of poorly differentiated squamous cell carcinoma [9]. Our study also showed higher PCNA index in poorly differentiated oral squamous cell carcinoma compared to well differentiated oral squamous cell carcinoma [Table/Fig-1].
Shin DM et al., analysed the dysregulation of PCNA in oral squamous cell carcinoma. The total increase in PCNA expression ranged from four fold to 10-fold from adjacent normal epithelium to squamous cell carcinoma [10]. Our study showed 3 to 4 fold increase in PCNA values in different grades of oral squamous cell carcinoma compared to normal epithelium. The difference in PCNA index value may be due to the difference in the clinical stage of oral premalignancy and malignancy. S Störkel et al., investigated cases of oral squamous cell carcinoma, and reported that the amount of antigen expression increases with the increasing grade of malignancy of the oral squamous cell carcinoma [11]. Our study also showed increasing PCNA values with increasing grades of malignancy [Table/Fig-1].
Girod SC et al., on quantitative analysis showed a gradual increase in PCNA expression from normal mucosa to moderately differentiated squamous cell carcinoma [12]. Our study also showed a similar pattern of increase in PCNA index [Table/Fig-1,3–7].
Zain RB et al., described about the different nuclear staining patterns for PCNA in normal, hyperplastic and dysplastic epithelium, early cancer and squamous cell carcinoma of the oral cavity and he reported that the expression of PCNA in all the grades of squamous cell carcinoma was present at the deep, infiltrative margins [13]. In our study hyperplastic epithelium was not included, as we intended to evaluate the PCNA uptake by cells of premalignant and malignant lesions.
Lan H A et al., assessed the intensity of immunoreactivity of PCNA and correlated it with tumour differentiation, nuclear atypia and the patterns of invasive margins in the underlying connective tissue. Increased expression of PCNA was indicative of poor differentiation, higher nuclear atypia and more invasive growth of tumour cells [14]. In our study also we observed poorer differentiation and higher nuclear atypia in tumour cells of poorly differentiated squamous cell carcinoma [Table/Fig-7,8].
Lehadh M Al-Azzawi reported that the presence of a high degree of PCNA proteins in chronic and premalignant lesions like oral lichen planus and epithelial dysplasia can be helpful in their prognosis and suggested treatment [15].
Conclusion
The present study was aimed to evaluate the immunohistochemical expression of PCNA in routine biopsies of oral pre malignant lesions and oral squamous cell carcinomas. Scores of positively stained PCNA cells were seen in an ascending order from normal to premalignant lesions and oral squamous cell carcinomas, suggesting an increase in the proliferative activity of the tumours cells. It can be concluded that PCNA index along with clinical features can be used for predicting aggressiveness and recurrence rate in these lesions.
p-value <0.05 is considered significant
p=0.001
Group I - Normal
Group II - Leukoplakia
Group III - Well differentiated Squamous cell carcinoma
Group IV - Moderately differentiated Squamous cell carcinoma
Group V - Poorly differentiated Squamous cell carcinoma
p-value <0.05 is considered significant
p=0.001