Major depressive disorder (MDD) is a mental disorder characterized by episodes of depressed mood, loss of interest or pleasure, feeling of guilt or low self-esteem, loss of energy, altered sleep patterns and difficulty in concentration. The life time prevalence of major depressive disorder (MDD) is approximately 17% [1]. MDD has been estimated to be the fourth major cause of disability worldwide, and may become second only to cardiovascular diseases by around 2020 [2]. The introduction of SSRI’s (Selective serotonin reuptake inhibitors) for the treatment of major depressive disorder has transformed clinical psychopharmacology. SSRI’s prescriptions in the US alone occur at a rate of six prescriptions per second, 24/7, year around [3]. Current antidepressant drugs for MDD have some limitations in efficacy and effectiveness, associated with undesirable effects which may account for poor drug compliance in patients. In this scenario there is a need for better management of depression and for further development of more selective and new-targeting drugs. Agomelatine a new antidepressant approved in many countries (Australia, Brazil, Russia, South Africa and Turkey) outside US has agonist action at MT1, MT2 receptors and antagonist action at 5-HT2C receptors [4]. The drug was approved in India by Central Drugs Standard Control Organization (CDSCO) on 10th September 2012 for the treatment of major depression in adult patients aged between 18–65 years [5]. This novel antagonist property at 5-HT2C receptors stimulates dopamine and nor-epinephrine release in prefrontal cortex and has procognitive but particularly antidepressant action in animals [6]. Therefore it is proposed to verify whether the new antidepressant Agomelatine with novel mechanism is more effective than Escitalopram in the treatment of MDD.
Materials and Methods
A prospective study was conducted at Outpatient Department of Psychiatry, GSL medical college & General hospital, Rajahmundry from 1-4-2013 to 31-10-2014, with each participant followed-up for a period of eight weeks. Institutional ethical committee approval was obtained before initiating the study. Informed consent was obtained from eligible patients before participating in the study and they were randomly allocated using random number table to either Agomelatine or Escitalopram group.
Selection Criteria
Inclusion Criteria
Newly diagnosed patients of either sex aged between 18 to 65 years who met the DSM-IV TR [7] criteria for major depressive disorder (MDD), single or recurrent major depressive episode, without psychotic features were included in this study.
Exclusion Criteria
Subjects with depressive disorder due to general medical condition, marked suicidal risk, bipolar disorder, schizophrenia, OCD, anxiety, psychoactive substance abuse or dependence within 1 year period to enrollment, pregnant and lactating mothers and those with other axis –I & II disorders were excluded.
At the screening visit the participants were subjected to detailed psychiatric and medical interview, those who fulfilled the selection criteria were explained in detail about the nature of the study, its purpose, procedure and follow-up. They were randomly assigned Agomelatine 25-50 mg/day or Escitalopram 10- 20 mg/ day for 8weeks with every two weeks follow-up. In case of unsatisfactory improvement using predefined criteria, the dosage of Agomelatine was increased to 50 mg/day after two weeks, and that of Escitalopram to 20 mg/day after two weeks. The efficacy of study medication on MDD was assessed at every 2-weekly visit using the HAM-D17 score (Hamilton depression rating scale) [8], CGI-S and CGI-I scores (Clinical Global Impression rating scales) [9]. The efficacy outcome considered was reduction in HAM-D17 total score from baseline to end of therapy. Secondary outcome measures were assessed using the CGI-S (Clinical Global Impression-Severity scale) to check for severity of psychiatric condition from baseline to end of therapy. While the CGI-I score (Clinical Global Impression-Improvement scale) was assessed from the second week. Responders were defined as those showing a decrease of ≥ 50% in Hamilton-D17 total score from baseline and remitters are those with a score less than 7 on Hamilton-D17. Laboratory investigations and ECGs were performed at baseline and at the end of week 8. Adverse events reported by the patients and those observed during the study were recorded.
Statistical Analysis
All statistical analysis was performed by using MS excel -2007 and SPSS software trial version 16. Quantitative variables were expressed by mean±SD and qualitative variables were expressed by percentages. Student t-test was used for comparing the groups and chi-square test was used for assessing the qualitative variables. For all statistical analysis p<0.05 was considered statistically significant.
Results
Out of 120 patients enrolled, 106 completed the study and 14 lost to follow up (8 from the Agomelatine group and 6 from the Escitalopram group). [Table/Fig-1] depicts the demographic data of the patients in each group. Both the groups were comparable in demographic characteristics and disease characteristics. The mean number of depressive episodes was 2.57±1.17 in Agomelatine and 2.48±1.09 in Escitalopram group with a median five months of depressive episode duration in both the groups. There was no statistically significant difference in various demographic characteristics like age, sex, education, marital status, employment status, illness duration and severity among the treatment groups.
Baseline demographic and clinical characteristics of patients
| Characteristics | Agomelatine n (52) | Escitalopram n (54) | p-value |
|---|
| Age (years) Mean±SD | 40.98±8.16 | 41.22±7.88 | p= 0.876 |
| Sex n (%) |
| Males | 25 (48.07) | 26 (48.18) | p= 0.99 |
| females | 27 (51.92) | 28 (51.85) |
| Marital status n (%) |
| Married | 30 (57.69) | 35 (64.81) | p=0.797 |
| Widow/Widower | 8 (15.38) | 6 (11.11) |
| Divorced / Separated | 12 (23.07) | 10 (18.51) |
| Unmarried | 2 (3.84) | 3 (5.55) |
| Education n (%) |
| Literate | 36 (69.23) | 41 (75.92) | p=0.439 |
| Illiterate | 16 (30.76) | 13 (24.07) | |
| Employment status |
| Employed | 29 (55.76) | 32 (59.25) | p=0.836 |
| Unemployed | 20 (38.46) | 18 (33.33) | |
| Retired | 3 (5.76) | 4 (7.40) | |
| DSM-IV Diagnosis n (%) |
| Single episode | 9 (17.3) | 10 (18.51) | p=0.87 |
| Multiple episode | 43 (82.69) | 44 (81.48) | |
| Depressive episodes Mean±SD | 2.57±1.17 | 2.48±1.09 | p=0.89 |
| Present depressive episode duration before inclusion (Months) Median | 5.0 | 5.0 | |
| HAM-D Total score Mean±SD | 27.26±2.92 | 27.18±3.19 | p=0.88 |
| CGI-S Mean±SD | 5.01±1.03 | 4.98±1.03 | p=0.85 |
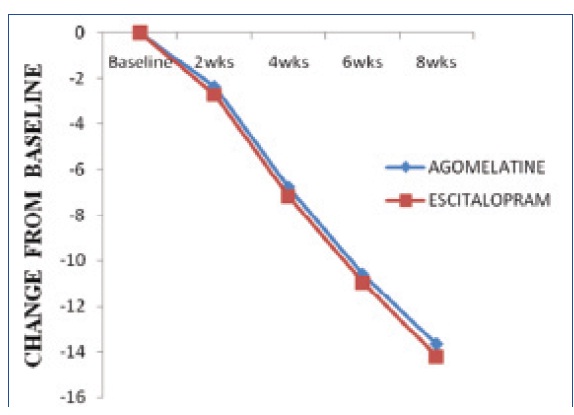
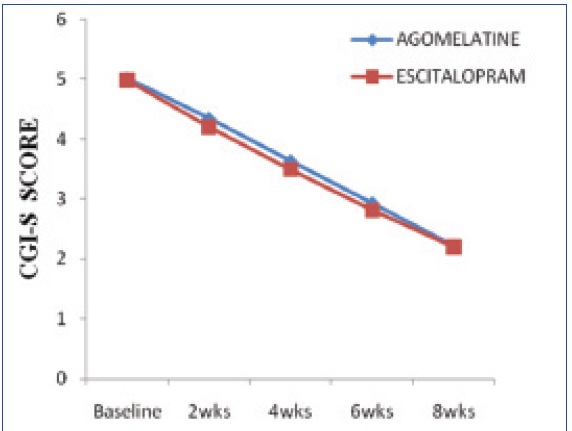
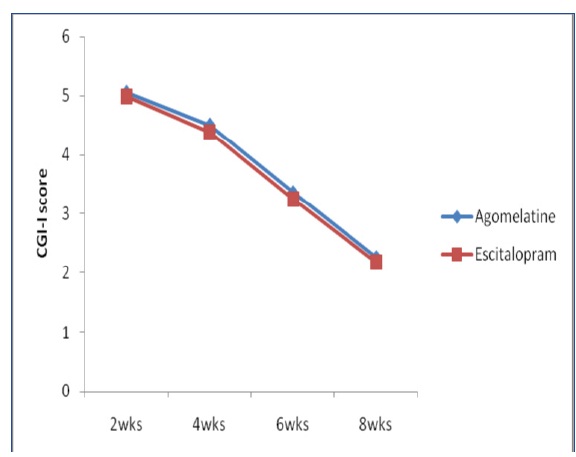
[Table/Fig-2] Represents HAMD-17 score showed a significant fall from treatment initiation at biweekly assessment in both treatment groups. However, the difference between groups was statistically not significant at any assessment of entire study period (p>0.05). But within the group after eight weeks of treatment the mean HAMD-17 total score decreased from baseline 27.26±2.92 and 27.18±3.19 to 13.58±5.42 and 12.94±4.48 with Agomelatine and Escitalopram respectively, which is statistically found to be significant (p<0.0001). The percentage of responders after 8weeks (last post baseline value) was 65.38% with Agomelatine and 57.40% with Escitalopram. The percentage of remitters after 8weeks was 21.02% with Agomelatine group and 22.22% with Escitalopram group. The mean CGI-S and CGI-I scores were decreased in both groups [Table/Fig-3,4] and there was no statistically significant difference between groups at any assessment of the study period (p>0.05). The severity of illness CGI-S score decreased from 5.02±1.03 and 4.98±1.03 at baseline to 2.21±0.66 and 2.20±0.78 with Agomelatine and Escitalopram respectively, which is found to be statistically significant (p<0.0001). In each treatment group the assessment of CGI-I score showed a statistically significant improvement at any point of study (p<0.0001). The CGI-I score at week 2 decreased from 5.06±0.57 and 4.98±0.73 to 2.27±0.49 and 2.19±0.43 with Agomelatine and Escitalopram respectively.
No serious adverse events were reported by any of the patients in both treatment groups. The percentage of patients reporting adverse events was 71 % in the Agomelatine group and 61% in the Escitalopram group. Most of the adverse events were mild in severity [Table/Fig-5]. The laboratory investigations and ECGs performed before initiation of treatment and at the end of therapy, did not show any clinically significant change from treatment initiation in both treatment groups.
Represents change in mean difference of HAMD-17 score, showed a significant fall from treatment initiation at biweekly assessment in both treatment groups
Mean change of CGI-S scores from baseline to the end of 8th week
Mean change of CGI-I scores from baseline to the end of 8th week
| Adverse event | Agomelatine (n=52) n (%) | Escitalopram (n=54) n (%) |
|---|
| Head ache | 5 (9.6) | 3 (7.4) |
| Nausea | 7 (13.44) | 7 (12.96) |
| Vomiting | 4 (7.68) | 3 (5.55) |
| Upper abdominal pain | 3 (5.76) | 4 (7.4) |
| Dry mouth | 4 (7.68) | 3 (5.55) |
| Diarrhea | 2 (3.84) | 2 (3.70) |
| Dizziness | 4 (7.68) | 2 (3.70) |
| Insomnia | 3 (5.76) | 4 (7.4) |
| Constipation | 1 (1.92) | 1( 1.85) |
| Fatigue | 2 (3.84) | 3 (5.55) |
| Dyspepsia | 2 (3.84) | 1 (1.85) |
| Patients without Adverse events | 15 (28.84) | 21 (38.88) |
Discussion
The main aim of the study was to compare the efficacy and safety profile of Agomelatine with Escitalopram in the treatment of MDD. The results of this study showed that Agomelatine is therapeutically similar to Escitalopram in terms of antidepressant efficacy. A regulatory biological clock mechanism is resided in the SCN (suprachiasmatic nucleus) which regulates circadian rhythms in the central nervous system through Melatonergic and 5HT2c receptors. The stability of internal and external phase relationships is hypothesised to be essential for a stable ‘normal’ mood state. Desynchronisation of internal rhythms plays an important role in the pathophysiology of depressive disorders and contributes to chronobiological susceptibility to depression [10]. Agomelatine is one of the novel drugs in the antidepressant category with unique mechanism of action. It has MT1 and MT2 agonist and 5-HT2c antagonist properties with pro- chronobiological effect [11]. It is an analogue of melatonin with high binding affinity to MT1 and MT2 G protein coupled melatonergic receptors [12]. These melatonergic receptors are expressed in various areas of the central nervous system, including SCN, cerebellum, hippocampus, central dopaminergic pathways, substantia nigra, ventral tegmental area, nucleus accumbens, amacrine and ganglion cells [13]. Agomelatine dose dependently inhibits the firing rate of SCN neurons and resynchronize circadian rhythms. The 5-HT2c antagonism has the ability to promote the dopaminergic firing at the ventral tegmental area, frontal cortex, hypothalamus, hippocampus, medulla, pons and also enhance the norepinephrinergic activity at the locus coeruleus [14,15]. The antidepressant effects of agomelatine are claimed to derive from a synergism between its melatonergic agonist and 5-HT2C antagonist actions [16]. The other study drug Escitalopram, selective serotonin reuptake inhibitor (SSRI), is the S-enantiomer of racemic citalopram. It binds to the serotonin transporter (SERT), induces a conformational change in the transporter which decreases the serotonin reuptake. This results in the enhancement of the synaptic availability of serotonin and improves the serotonergic function in the central nervous system [17]. It has an additional modulatory effect at allosteric binding site on the serotonin transporter protein which increases the binding time of the primary site, resulting in greater inhibition of 5-HT reuptake by the 5-HT transporter [18]. The present study showed that the percentage of responders at the end of week 8 was 65.38% with Agomelatine and 57.40% with Escitalopram group (HAMD-17). There was a notable percentage of remitters at the end of study period, 26.92% and 24.07% (CGI-I) with agomelatine and escitalopram respectively. In the responder analysis, a difference between the two groups in response rates of 7.98 (HAMD-17) and 2.85 (CGI-I) percentage points was observed. There are few clinical trials which evaluated efficacy and safety of agomelatine in MDD.
Quera-Salvaet et al., conducted a clinical trial that showed that the percentage of responders at the end of week 6 was 69% with Agomelatine and 59% with Escitalopram [19]. After 24 weeks, the rate of responders is 77% and 74% and the rate of remitters is 48% and 42% with agomelatine and escitalopram respectively. Another study which is a 24-wk randomized, controlled, double blind trial by Emmanuelle Corruble et al., showed that the percentage of responders at the end of week 12 was 83.6%and 79.7% with agomelatine and escitalopram respectively [20]. After 24 weeks, the rate of responders is 82% and 79.7% with agomelatine and escitalopram respectively. The percentage of remitters over both the 12wk and 24-wk treatment periods was 55.7% and 65.6% in the agomelatine group where as 52.3% and 62.5% in the escitalopram group. The present study findings showed a similar rate of response to treatment on both HAMD-17 total score and CGI-I scales at week 6 and week 12 of two previous clinical trials but differs in findings at week 24. The difference observed with the previous studies was better improvement in those on long term therapy.
Remission is uncommon in short-term studies, because it often occurs beyond the study period [21]. Adverse effects were considered responsible for poor patient compliance to antidepressant treatment [22]. In the present study the incidence of adverse events were minimal and equally low for agomelatine and escitalopram, they were nonserious and did not require dose modification or discontinuation of the drug therapy. Gastrointestinal system related adverse events were frequently reported in both the groups, less frequently noted adverse events were headache (9.6% vs 7.4%), dizziness (7.68% vs 3.70%), insomnia (5.6% vs 7.4%) and fatigue (3.48% vs 5.55%) for agomelatine and escitalopram respectively. Hence, the present study confirms the good tolerability of agomelatine. This is in line with other studies on agomelatine [21,23–25].
Limitations of the Study
Eight weeks is a short period to comment on the safety and efficacy of the study drugs and small sample size is another limitation which recommends that further studies with large sample size is needed to throw light regarding the efficacy and safety of the agent.
Conclusion
Based on the treatment outcome, the study suggests that Agomelatine 25–50 mg /day is effective and as safe as Escitalopram 10–20 mg/day in the short-term treatment. During the study period both the drugs were similarly resolving depressive symptoms in Major depressive patients there by Agomelatine is considered therapeutically similar to Escitalopram in the treatment of Major depressive patients.