Preliminary Experience and Morbidity Analysis of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC) from a Tertiary Cancer Center in India
Naveen Padmanabhan1, Barath Raj Kumar2, Ansar Pullampara Pookunju3, Ayyapan Srinivasan4, Vikash Mahajan5
1 Registrar, Department of Surgical Oncology, Apollo Speciality Hospitals, Chennai, India.
2 Registrar, Department of Surgical Oncology, Apollo Speciality Hospitals, Chennai, India.
3 Senior Registrar, Department of Surgical Oncology, Apollo Speciality Hospitals, Chennai, India.
4 Head and Senior Consultant, Department, of Surgical Oncology, Apollo Speciality Hospitals, Chennai, India.
5 Senior Consultant, Department of Surgical Oncology, Apollo Speciality Hospitals, Chennai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Naveen Padmanabhan, W-513, 9th Street, Sector-C, Annanagar West Extension, Chennai-600101, India. E-mail : drnaveenp.in@gmail.com
Background
Peritoneal carcinomatosis (PC) can arise directly from peritoneum (primary) or from regional spread of gastrointestinal and gynecological malignancies. It is often considered a terminal event. CRS/HIPEC procedure provides encouraging outcomes in select sub-set of patients with PC. In this study we present our initial experience of this combined procedure from a tertiary cancer care center in India.
Materials and Methods
Between January 2014 to January 2015, 13 patients underwent CRS + HIPEC procedure at our center. Preoperative assessment for cytoreduction was done using contrast CT-scan of the abdomen and staging laparoscopy. All procedures were performed by the same surgical team. After cytoreduction, HIPEC was performed by closed method.
Results
Median patient age was 52 and median PCI was 13.5 (5-21). Ovarian cancers were commonest origin of PC in our series. All patients had a complete cytoreduction with a median operative time of 8.3 hours. Postoperative ileus was the commonest adverse event. In the immediate postoperative period, major complications were observed in 23% (3/13) of our patients (1. intra-abdominal abscess 2. Septicemia and liver function derangement 3. Bowel obstruction which required a re-operation. Median hospital stay was 12 days (range 9-45 days) and there was no perioperative mortality.
Conclusion
Our initial results indicate that CRS + HIPEC procedure can be performed with acceptable morbidity and no mortality. Appropriate case selection by a multi-disciplinary team is vital to achieve complete cytoreduction and optimize outcomes.
Carcinomatosis, Colorectal neoplasm, Ovarian neoplasm, Regional chemoperfusion
Introduction
Peritoneal carcinomatosis (PC) represented by presence of tumour deposits in parietal and visceral peritoneum, commonly arises from regional spread of gastro-intestinal or gynaecological malignancies. It can also originate denovo from peritoneum as in primary peritoneal serous carcinoma (PPSC) or mesothelioma. The outcome of PC has been uniformly poor irrespective of the site of origin with survival less than 12 months [1,2]. Traditionally it was considered to be surgically incurable disease with surgery being reserved for tumour complications like bleeding, bowel obstruction or perforation. The disease causes considerable compromise in the quality of life with complications like pain, ascites, intractable and repeated bowel obstructions and bleeding. Furthermore palliative surgeries offered to improve these symptoms often fail. Paul Sugarbaker from Washington cancer institute in 1990s introduced extensive cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) a novel treatment option for selected patients with PC [3]. The procedure involves aggressive cytoreduction with multiorgan resection and peritonectomy to remove all visible macroscopic disease and infusion of heated chemotherapeutic agent in the abdominal cavity to eradicate the microscopic tumour residues. Over the past three decades many observational studies and one randomized control trial has proved the long term survival benefit of CRS and HIPEC in PC. Most literature about CRS and HIPEC come from high volume tertiary centers while some studies report their initial experience with the procedure [4]. Since the procedure is associated with high morbidity it might be useful to discuss the experience of newly initiated setups thereby promoting safety of procedure in similar settings. In this article we present our initial experience of 13 cases in our setting and aim to discuss the safety, feasibility and postoperative complications of CRS+HIPEC procedure from a single institution cohort.
Materials and Methods
This is a prospective observational study of CRS/HIPEC procedures performed at our centre. Institutional ethical committee clearance was obtained and informed consents for the study were taken from all patients who underwent the procedure. Between January 2014 to January 2015, 13 patients underwent CRS+HIPEC procedure at our center. All procedures were performed by the same surgical team. Inclusion criteria were clinical diagnosis of PC, good performance status (Eastern cooperative oncology group score ≤2), no major co-morbidity, no extra-abdominal metastasis and disease amenable for complete cytoreduction based on Preoperative imaging that included Contrast CT of abdomen and pelvis to assess the disease extent and its operability. PET-CT was done to rule out extra-abdominal metastases.
Preoperative preparation
Routine blood examinations and cardiopulmonary assessment were performed. When features in CT scan suggest doubtful resectability, diagnostic laparoscopy was done to make a final decision. Neo-adjuvant chemotherapy (NACT) was started as clinically indicated. We counselled the patient and family members regarding the surgery, complications, potentially prolonged course of treatment schedule and the need for adequate nutrition.
Operative procedure
A generous midline laparotomy was used and tumour extent and distribution was scored using peritoneal carcinomatosis index (PCI) as described by Sugarbaker [Table/Fig-1]. Surgery was aimed at complete removal of visible macroscopic disease. Bowel, peritoneum and organ resections were performed as per tumour involvement and bowel anastomosis was done routinely after completion of HIPEC. Peritoneum uninvolved by tumour was left to be treated with HIPEC. After surgery extent of resection was assessed based on completeness of cytoreduction score [Table/Fig-1]. Prior to HIPEC phase thorough adhesiolysis was performed to ensure proper drug distribution to all abdominal quadrants. The abdomen is closed temporarily with running sutures to skin. HIPEC was performed using Exiper unit for locoregional therapy in oncology (Menfis division of Medica S.P.A). Circuit connections and perfusate preparation was done by the technical team. Two inflow and three outflow drain tubes (28 Fr) were placed in right and left quadrants of the abdomen respectively and thermal monitors were placed in pelvis and subdiaphragmatic region. Input and output catheters were connected to the HIPEC machine. About 3.5-4.5 liters of Baxter peritoneal dialysis solution (2.5% w/v dextrose) heated to 42-43°C was used for perfusion. Once the perfusion fluid achieves target temperature in the abdomen, chemotherapeutic agent was added into the circuit and flow rate was set at 800-1000 ml/hr. Two thirds of the dose was administered in the first half and the remaining one-third of the dose was added in second half. When oxaliplatin was used the total HIPEC time was limited to 30 minutes and for other agents the time was 90 minutes. Abdomen temperature was maintained between 42-43°C. Table was tilted in various positions and abdomen was manually rocked to ensure even drug distribution. Following HIPEC therapy abdomen was opened and thorough wash was given with normal saline at room temperature. Intra-peritoneal (IP) chemoport was placed in left lower chest wall in sub cutaneous plane and abdomen was closed. In the immediate Postoperative period all patients were managed in intensive care unit.
PCI and Completeness of cytoreduction score described by Sugarbaker [3]
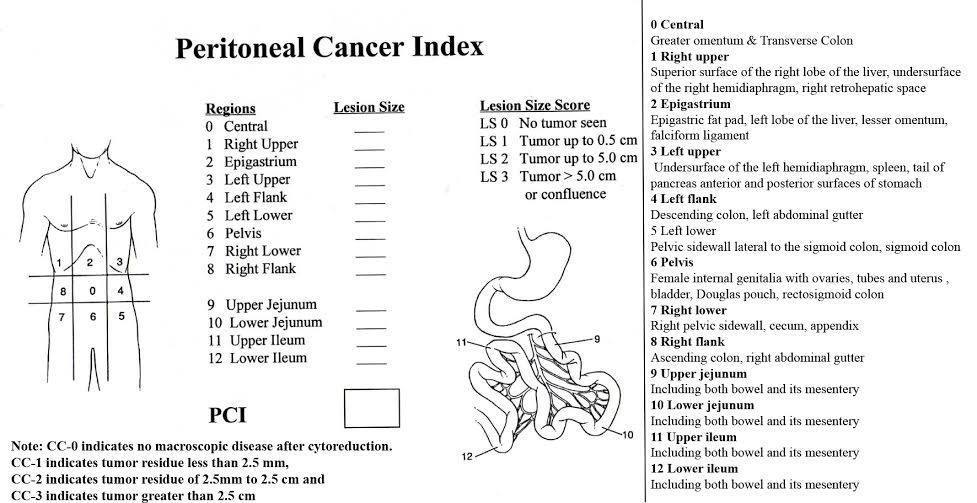
Data on Postoperative course, complications and follow-up were recorded. Complications were graded as per Common terminology National Institutes of Health Common ToxicityCriteria for adverse events (CTCAE) version 4.0. Grade I and II complications have mild to moderate symptoms requiring nil or non-invasive intervention. Grade III and IV have severe to life threatening symptoms requiring hospital admissions and major interventions. All patients were followed up regularly.
Results
Patient’s preoperative and intraoperative characteristics are elaborated in [Table/Fig-2]. All patients were symptomatic at presentation expect for one. One patient had bilateral complex ovarian masses picked up in ultrasound during master health check-up and subsequent investigations revealed ovarian tumour with PC. The median PCI index was 13.5 (range 5-21) and a score of 21 was obtained in mucinous neoplasm of appendix. [Table/Fig-3] describes the list of procedures performed in our patients. Nine patients with non-GI primary (ovarian PC and PPSC) required some form of bowel resection as a part of cytoreduction (4 anterior resections, 3 subtotal colectomies, 2 small bowel resections, 1 sigmoid resection and 1 hemicolectomy). Two patients had liver nodule excision. One amongst them was a recto sigmoid primary with multiple liver metastasis who had radiofrequency ablation of 2 lesions preoperatively and resection of 3 more lesions intraoperatively. Complete cytoreduction was achieved in all 13 cases (CCS 0) and HIPEC was subsequently done as described above. In the initial two patients ice packs were placed around the limbs for external cooling during HIPEC phase. This caused skin necrosis which was managed by regular dressings and antibiotics.
Pre-operative and intra-operative characteristics of patients who underwent CRS/HIPEC
| PATIENT CHARACTERSTICS | RESULTS |
|---|
| Median age (range) | 52.5 (39-64) |
| Primary Tumors | |
| Primary peritoneal carcinoma | 4 |
| Primary ovarian carcinoma | 2 |
| Recurrent ovarian carcinoma | 3 |
| Mucinous neoplasm of appendix | 1 |
| Appendix adenocarcinoma | 1 |
| Colorectal cancers | 2 |
| NACT received | 7 |
| Performance Score (ECOG SCALE) | |
| 0 | 1 |
| 1 | 5 |
| 2 | 7 |
| PCI index median(range) | 13.5 (5-21) |
| CCR score 0/1 | 13 |
| INTRA-OPERATIVE CHARACTERSTICS: | |
| Duration of CRS/HIPEC Median (range) | 8.33 (7.5-9) hrs |
| PRBC transfusion Median (range) | 1200 (850-1500) ml |
| FFP transfusion Median (range) | 600 (450-750) ml |
| Chemotherapy agent during HIPEC | |
| Mitomycin | 4 |
| Cisplatin | 6 |
| Cisplatin + Mitomycin | 2 |
| Oxaliplatin | 1 |
| Median of Max. Intra-abdominal temperature during HIPEC phase | 42 (42-44) °C |
| Median of Max. body temperature during HIPEC phase | 37.9 (37-39.6) °C |
List of procedures performed for cytoreduction
| Procedures | Ovarian (2) | Rec.Ovarian (3) | Colorectal (2) | Appendix (2) | PPSC (4) |
|---|
| Diagnostic laparoscopy | 1 | 3 | | 0 | 2 |
| TAH + BSO | 2 | | | 1 | 2 |
| Pelvic node dissection | 2 | | | 1 | 2 |
| Para-Aortic Node dissection | 2 | 3 | | | 2 |
| Colonic Resection | | | | | |
| Rt. Hemicolectomy | | | | 2 | 1 |
| Subtotal Colectomy | | 2 | 1 | | 1 |
| Sigmoid resection | 1 | | | | |
| Anterior Resection | 1 | 1 | 1 | 1 | 2 |
| Small Bowel Resection | | 1 | | | 1 |
| Splenectomy | | 2 | | 1 | |
| Liver Resection (Metastasectomy) | | 1 | 1 | | |
| Radiofrequency abalation of liver lesion | | | 1 | | |
| Cholecystectomy | | 2 | | | 2 |
| Pelvic Peritonectomy | 2 | 3 | | 1 | 2 |
| Rt Paracolic Peritonectomy | 2 | 3 | | 2 | 2 |
| Lt Paracolic Peritonectomy | 2 | 3 | | | 2 |
| Sub diaphragmatic peritonectomy | 2 | 3 | | 1 | 2 |
| Parietal Peritonectomy | | 3 | | | 2 |
| Mesh for Abdomen Reinforcement | | 2 | | 1 | |
| U/L ICD placement | 1 | 1 | | | |
| B/L ICD placement | 1 | 2 | | 2 | 1 |
| IP port Placement | 1 | 3 | | 1 | 2 |
Postoperative course is elaborated in [Table/Fig-4]. Two patients had only one adverse event (Ileus and atelectasis) whereas the other 9 had more than one adverse event. Most common observed complication was paralytic ileus (6 patients; 43%).
Post-operative course after CRS/HIPEC
| Parameter | Number (n) |
|---|
| Median TPN Requirement (range) | 3 (2-7) days |
| Median ICU stay (range) | 2 (2-4) days |
| Median requirement of parenteral analgesics (range) | 4 ( 3 - 5) days |
| Median time for initiating oral liquids (range) | 4 (4 – 7) days |
| Median time to resume normal diet (range) | 8 (7 – 15) days |
| Median Hospital stay (range) days | 12 (9-45) |
| Uneventful Recovery (n cases) | 2 |
| Immediate Post-op Events |
| Grade I/II complications (n patients) | 13 |
| Ileus | 6 |
| Respiratory complications* | 3 |
| Bladder dysfunction ** | 2 |
| Electrolyte Imbalance*** | 2 |
| Grade III/IV complications (n patients) | 3 |
| Intra-abdominal collection and septicemia | 1 |
| Septicemia, Deranged Liver function | 1 |
| Bowel obstruction | 1 |
* -1.atelectasis, 2.pnuemonia, 3.hypoxia.
**-1.Acute urinary retention and recatheterisation, 2.UTI, dysuria and associated incontinence.
***-1. Hypokalemia and hypomagnesemia, 2. Hypoalbuminemia.
Three grade III/IV complications (23%) were noted. One patient with rectal primary who had oxaliplatin in HIPEC developed prolonged ileus, aspiration pneumonia, bacterial and fungal septicemia, high output renal failure and deranged liver function which was managed by prolonged antibiotic therapy, fluid management and nutritional support. His hospital stay was prolonged to 45 days. The second patient developed intestinal obstruction during postoperative day 7-9 for which she underwent re-laparotomy and found to have adhesive obstruction of distal ileum. After the second surgery she had persistent fever that settled after IP port removal. Third patient had prolonged fever due to intra-abdominal abscess and septicemia necessitating image guided aspiration and parenteral antibiotic therapy. We did not observe any mortality, anastomotic leak, haematological or haemorrhagic complications in postoperative period. During the follow-up one patient developed sub-acute intestinal obstruction after 1st cycle of IP chemotherapy which settled over 15 days on conservative management. Length of follow-up ranged from 2.5 months to 14 months. One of the patient with recurrent ovarian tumour relapsed 10 months after the procedure with increasing CA-125 levels and upper abdominal cystic masses. She’s being managed with supportive care. Two other patients (primary ovarian tumour and appendiceal carcinoma) had rising tumour marker levels detected eight months after the procedure but in both patients imaging studies did not reveal any recurrence. Both are being actively followed-up and maintained on supportive care. The rest 10 patients are alive and recurrence free.
Discussion
The management of PC has evolved over the past two decades from one of non-intervention to extensive debulking surgery. First reported combined cytoreduction and HIPEC was by Spratt et al., [5] in 1980 wherein he treated a young pseudomyxoma patient using Thiotepa for IP chemoperfusion. In 1990s Paul Sugarbaker popularized the technique since then multiple studies have proved the efficacy of this integrated procedure in peritoneal carcinamatosis of various origins. Best results have been obtained in appendiceal psuedomyxoma peritonei (PMP) wherein the reported 10-year and 15-year survival rates are 63% and 59% respectively [6]. In several observational studies PC of colorectal, ovarian, primary peritoneal serous carcinoma (PPSC) and peritoneal mesothelioma origin have been treated with CRS+HIPEC offering 5 year survival rates of 20-40%, 63%, 57% and 47% respectively [7–11]. In PC of colorectal origin, a randomizedtrial by Verwaal et al., reported a statistically significant survival benefit of CRS+HIPEC over systemic chemotherapy (22.3 vs 12.6 months p=0.032) and subset of patients with complete cytoreduction had 45% five-year survival [8]. In contrast treatment of colorectal PC with modern systemic chemotherapeutic agents like oxaliplatin and irinotecan has yielded a mere 4.1% 5- survival rate [12]. Despite these promising results, the efficacy of this procedure continues to be debated due to its morbidity concerns. Nevertheless an increasing number of centers across the world are adopting this procedure for treatment of peritoneal carcinomatosis.
Cytoreductive surgery with HIPEC is a major surgical procedure often involving resection of multiple organs and confers major physiological changes in GI, respiratory and cardiac systems. High volume treatment centers report peri-operative morbidity rates of 25-41% and mortality rates of 0-5% [13]. Postoperative complications can be due to surgery or HIPEC and also likely to be cumulative. High PCI index, long duration of surgery, intraoperative blood loss greater than 2.5 liters, small bowel resection, increasing number of anastomoses and peritonectomy procedures and early phase of learning curve are some recognized risk factors. Commonly encountered complications are ileus, anastomotic leak, Postoperative bleeding, intra-abdominal collection, wound dehiscence and respiratory compromise [13–16].
Four cases with PCI <10 either had uneventful course or only grade I/II complications and two of three patients who developed grade III/IV complications had PCI > 15 [Table/Fig-5]. In our cohort the commonest observed complication was ileus followed by respiratory complications. Similar to us many studies have reported that respiratory complications like effusion, hypoxia, atelectasis and adult respiratory distress syndrome (ARDS) are the second most common adverse events after abdominal complications. Plueral effusion is common to occur after this procedure which could be reactive or due to opening of plueral space during sub-diaphragmatic peritoneal stripping [17]. To circumvent this issue we prophylactically inserted intercostal drain tube whenever diaphragmatic stripping was done. To limit the morbidity due to bowel resection, excision or electrofulgration of serosal deposit was performed whenever feasible.
Comparison of PCI and grade of complications after CRS/HIPEC
| PCI index | No adverse events | Grade I/II complications | Grade III/IV complications |
|---|
| ≤ 10 (n=4) | 2 | 2 | NIL |
| 10-15 (n=6) | Nil | 10 | 1 |
| > 15 (n=3) | Nil | 1 | 2 |
Adverse events related to HIPEC therapy is due to chemotherapeutic agent and hyperthermia. Haematological toxicity associated with HIPEC is reported to be less than 5% as IP chemotherapy has advantages of achieving high local concentrations for tumouricidal effects and limited systemic absorption due to plasma-peritoneal barrier [14]. Haematological toxicity increases when bi-directional chemotherapy or early intra-peritoneal chemotherapy is used. Neutropenia can particularly have profound effect on healing and can cause additional complications due to anastomotic leaks. However, we did not have any major haematologic complications in our patients. Hyperthermia has intrinsic and synergistic tumouricidal activity with agents like cisplatin and oxaliplatin [18–20]. During the HIPEC phase core body temperature (CBT) was monitored and care was taken to maintain it below 39.5°C. Methods employed were cooled IV fluid transfusion, tepid sponging and reduction of ambient theatre temperature. Ice packs placed around limbs for cooling could exacerbate the peripheral vasoconstriction and result in skin damage as observed in our study.
Main disadvantage of performing HIPEC by closed technique is improper drug distribution and consequently pooling of drug in isolated areas contributing to focal hyperthermic injury. However, its advantage lies in minimal exposure of chemotherapy agents to theatre personnel and safe disposal of chemo agent back to the circuit in a closed system. We attempted open technique initially and experienced difficulties in maintaining the desired intra-abdominal temperature. In subsequent cases we were able to safely perform HIPEC by closed technique as reported in several other centers [21].
The process of appropriate case selection and preoperative preparation is vital for successful outcome. The preoperative evaluation of patients considered for the procedure includes assessment of extent of disease to determine resectability and assessment of cardiopulmonary reserve to ascertain if the intended resection can be performed with acceptable perioperative risk. Cases with PCI more than 20 are not considered for CRS/HIPEC procedure. Presence of ascites, bowel obstruction at more than two sites, porta-hepatis involvement and parenchymal liver involvement are certain features that can lead to incomplete cytoreduction and increased morbidity. Presence of at least one third of small bowel after resection is a prerequisite for adequate bowel function [22]. Old age is not considered an absolute contraindication when patients have good performance status [23]. Preoperative preparation with particular care to optimize nutrition and respiratory reserve is necessary. It is common to find malnourishment at presentation due to catabolic effect of advanced tumour and poor intake due to bowel dilation. Recommendations have been made to consider appropriate nutritional intervention in the peri-operative period [24]. Accordingly Preoperative nutritional supplements were initiated and all patients received Postoperative parenteral nutrition, as enteral feeding is commonly delayed due to presence of multiple bowel anastomoses.
Achieving complete cytoreduction is the single most important factor for obtaining long term survival benefit. In our early experience we report a 100% CC-0 score in 13 patients probably due to stringent and cautious selection process. Despite adequate cross sectional imaging about 20-40% of patients become inoperable due to inaccurate assessment of small bowel by CT scan [25,26]. Considering that such non-therapeutic laparotomies are associated with high morbidity (12-23%) and mortality (20-36%) [27], diagnostic aparoscopy (DL) has been advocated for initial evaluation. However, the use of laparoscopy has not gained much popularity as adhesions from prior surgeries and dilated bowel loops often cause technical difficulties in port positioning and adequate visualization of all abdominal quadrants. Nevertheless authors have previously reported successful laparoscopic staging in PC with minimal complications [28,29]. The prime advantage of laparoscopy lies in evaluation of small bowel, better assessment of operability and possibility of complete evaluation of all 13 quadrants to assess true PCI. Ineligible patients can be started on neoadjuvant chemotherapy and second look DL can be done for response assessment. We did diagnostic laparoscopy in 6 of our patients and started them on NACT as complete cytoreduction would not have been possible.
Establishing a new PC treatment facility poses a considerable challenge; committed surgical team and anesthesiologist with specific experience in long duration surgeries, availability of HIPEC machine, appropriate patient selection by a multi-disciplinary team, education of ancillary staff on the procedure and its complications are some of the prerequisites for initiating a treatment facility. Phase of learning curve influences clinical outcomes as it has been demonstrated that perioperative complications and rates of incomplete cytoreduction decrease with increasing expertise. A major PC treatment center in Italy, before initiating the facility had their senior surgeons assist over 40 procedures in well-known European centers and undergo fellowship in Washington Cancer Institute [30]. In our view the learning curve is more associated with cytoreductive component involving multi-organ resections and peritonectomies. Senior authors (Author number-4 & 5) in our center have vast experience in extensive cytoreductive surgeries in ovarian tumours which encouraged us to confidently embrace the approach. Before starting the treatment facility, senior authors did an advanced course in HIPEC conducted by European Society of Surgical Oncology in Hamburg, Germany and underwent observership in several other European centers.Also in our opinion surgery by team involving two surgeons of parallel experience can be vital in reducing the operating time and could possibly contribute to better performance.
Finally our study is limited by sample size and heterogeneous etiology of peritoneal carcinomatosis. Survival analysis is not possible in our study due to limited follow-up. Nevertheless our results reveal that Grade III/IV complication rates are identical to other major abdominal surgeries that could be managed with minimal mortality risk. Many physicians continue to have reservations about the benefit of the procedure and refrain from referring these patients for surgical assessment. Referral at later stages of disease is unlikely to be curative or offer long term survival benefit. Hence cases of PC must be discussed in multi-disciplinary boards to assess and refer them for CRS+HIPEC procedure in timely manner.
Conclusion
Our initial results indicate that CRS+HIPEC procedure can be performed with acceptable morbidity and no mortality. Committed surgical team with adequate experience in extensive cytoreductive surgeries is an essential pre-requisite. Diagnostic laparoscopy is a useful adjunct to decrease the rates of inoperability and incomplete cytoreduction. Careful case selection by MDT is necessary to optimize the outcomes.
* -1.atelectasis, 2.pnuemonia, 3.hypoxia.
**-1.Acute urinary retention and recatheterisation, 2.UTI, dysuria and associated incontinence.
***-1. Hypokalemia and hypomagnesemia, 2. Hypoalbuminemia.
[1]. Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective studyCancer 2000 88(2):358-63. [Google Scholar]
[2]. Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC, Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factorsCancer 1989 63(2):364-67. [Google Scholar]
[3]. Sugarbaker PH, Peritonectomy proceduresAnn Surg 1995 221(1):29-42. [Google Scholar]
[4]. Konstantinidis IT, Young C, Tsikitis VL, Lee E, Jie T, Ong ES, Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion: The University of Arizona early experienceWorld J Gastrointest Surg 2012 4(6):135-40. [Google Scholar]
[5]. Spratt JS, Adcock RA, Muskovin M, Sherrill W, Mckeown J, Clinical delivery system for intra-peritoneal hyperthermic chemotherapyCancer Res 1980 40(2):256-60. [Google Scholar]
[6]. Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Early-and long-term outcome data of patients with pseudomy xoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapyJ ClinOncol 2012 30(20):2449-56. [Google Scholar]
[7]. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancerJ Clin Oncol 2003 21(20):3737-43. [Google Scholar]
[8]. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H, 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancerAnn Surg Oncol 2008 15(9):2426-32. [Google Scholar]
[9]. Bijelic L, Jonson A, Sugarbaker PH, Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancerAnn Oncol 2007 18(12):1943-50. [Google Scholar]
[10]. Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experienceJ Clin Oncol 2009 27(36):6237-42. [Google Scholar]
[11]. Bakrin N, Gilly FN, Baratti D, Bereder JM, Quenet F, Lorimier G, Primary peritoneal serous carcinoma treated by cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy. A multi-institutional study of 36 patientsEur J Surg Oncol 2013 39(7):742-47. [Google Scholar]
[12]. Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841J Clin Oncol 2012 30(3):263-67. [Google Scholar]
[13]. Ahmed S, Oropello JM, Critical care issues in oncological surgery patientsCrit Care Clin 2010 26(1):93-106. [Google Scholar]
[14]. Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen techniqueCancer 2006 106(5):1144-53. [Google Scholar]
[15]. Chua TC, Saxena A, Schellekens JF, Liauw W, Yan TD, Fransi S, Morbidity and mortality outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy at a single tertiary institution: towards a new perspective of this treatmentAnn Surg 2010 251(1):101-6. [Google Scholar]
[16]. Kusamura S, Baratti D, Deraco M, Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignanciesAnn Surg 2012 255(2):348-56. [Google Scholar]
[17]. Preti V, Chang D, Sugarbaker PH, Pulmonary Complications following Cytoreductive Surgery and Perioperative Chemotherapy in 147 Consecutive PatientsGastroenterol Res Pract 2012 2012:635314[Internet]. doi: 10.1155/2012/635314 [Google Scholar]
[18]. Sugarbaker PH, Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapyInt J Hyperthermia 2007 23(5):431-42. [Google Scholar]
[19]. van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell linesEur J Cancer 1998 34(1):148-54. [Google Scholar]
[20]. Rietbroeck RC, van de Vaart PJ, Haveman J, Blommaert FA, Geerdink A, Bakker PJ, Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cellsJ Cancer Res Clin Oncol 1997 123(1):6-12. [Google Scholar]
[21]. Loggie BW, Fleming RA, McQuellon RP, Russell GB, Geisinger KR, Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of gastrointestinal originAm Surg 2000 66(6):561-68. [Google Scholar]
[22]. Cotte E, Passot G, Gilly FN, Glehen O, Selection of patients and staging of peritoneal surface malignanciesWorld J Gastrointest Oncol 2010 2(1):31-35. [Google Scholar]
[23]. Spiliotis JD, Halkia E, Boumis VA, Vassiliadou DT, Pagoulatou A, Efstathiou E, Cytoreductive Surgery and HIPEC for Peritoneal Carcinomatosis in the ElderlyInt J Surg Oncol 2014 2014:987475[Internet] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4009200/pdf/IJSO2014-987475.pdf [Google Scholar]
[24]. Vashi PG, Gupta D, Lammersfeld CA, Braun DP, Popiel B, Misra S, The relationship between baseline nutritionalstatus with subsequent parenteral nutrition andclinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapyNutr J 2013 12:118 [Google Scholar]
[25]. Iversen LH, Rasmussen PC, Laurberg S, Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosisBr J Surg 2013 100(2):285-92. [Google Scholar]
[26]. Pomel C, Appleyard TL, Gouy S, Rouzier R, Elias D, The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapyEur J Surg Oncol 2005 31(5):540-43. [Google Scholar]
[27]. Esquivel J, Farinetti A, Sugarbaker PH, Elective surgery in recurrent colon cancer with peritoneal seeding: when and when not to proceed. [Article in Italian]G Chir 1999 20(3):81-86. [Google Scholar]
[28]. Valle M, Garofalo A, Laparoscopic staging of peritoneal surface malignanciesEur J Surg Oncol 2006 32(6):625-27. [Google Scholar]
[29]. Jayakrishnan TT, Zacharias AJ, Sharma A, Pappas SG, Gamblin TC, Turaga KK, Role of laparoscopy in patients with peritonealmetastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC)World J Surg Oncol 2014 12:270 [Google Scholar]
[30]. Kusamura S, Baratti D, Hutanu I, Rossi P, Deraco M, The Importance of the Learning Curve and Surveillance of Surgical Performance in Peritoneal Surface Malignancy ProgramsSurg Oncol Clin N Am 2012 21(4):559-76. [Google Scholar]