Introduction
Psychosis is a symptom of mental illness characterized by a distorted or non-existent sense of reality. Schizophrenia is a severe mental illness characterized by positive symptoms (hallucinations, delusions, disorganized speech and agitated behaviour), negative symptoms (apathy, avolition, alogia) and cognitive deficits [1]. It has a worldwide prevalence of 1%, while the prevalence in India is 2.3/1000 population [2].
Antipsychotics are drugs used in the treatment of psychoses. The choice of antipsychotic drugs for long term schizophrenia treatment is based primarily on avoiding the adverse effects and improving the compliance [1]. Typical antipsychotics are associated with high rates of adverse effects like acute extra pyramidal signs and tardive dyskinesia that leads to poor compliance and thereby increasing the chances of relapse [3]. The atypical antipsychotics, on the other hand, shows modest therapeutic superiority compared to typical antipsychotics in positive, negative, cognitive and mood symptoms and also have lower risk of extra pyramidal adverse effects, which improves patient compliance, but the greatest area of concern with the atypical antipsychotics are the metabolic side effects [1,4]. They induce changes in weight, but they vary in their ability to induce such changes [1]. It has also been demonstrated that antipsychotic-induced weight gain is the main cause of non-compliance and discontinuation of treatment, often resulting in the relapse of psychosis [5]. In a comprehensive review of research literature, it was found that both conventional and newer antipsychotics are associated with weight gain. Among them, atypical antipsychotics appear to have the greatest potential to induce weight gain [6].
Amisulpride is an atypical antipsychotic which is a highly selective dopamine D2-like receptor antagonist. It binds selectively to dopamine D2 and D3 receptors in the limbic system [7]. It is effective for both positive and negative symptoms of schizophrenia [8] and has a lower propensity to cause weight gain [9]. Blonanserin is a novel atypical antipsychotic which acts as an antagonist at dopamine D2, D3 and serotonin 5-HT2A receptors [10]. Its affinity for D2 receptors is approximately 6 times greater than that for 5-HT2A receptors. This property of blonanserin differentiates it from other atypical antipsychotics [11]. It is also effective for both positive and negative symptoms of schizophrenia and is generally well tolerated and appears to have an acceptable side effect profile [12].
There are very little head to head comparison between the weight gain associated with newer atypical antipsychotics in general and between amisulpride and blonanserin in particular. Clinical studies would be of great help to assess the weight gain associated with blonanserin, in comparison with amisulpride, which with prior clinical trials have shown to have lesser metabolic side effects.
Materials and Methods
Trial design: Open labeled, randomized controlled trial, done between 1st December 2012 – 31st March 2014.
Sample size: Sample size was calculated considering standard deviation (SD) of increase in weight gain in amisulpride as 1.56 [13] and in blonanserin as 1.55 [12,13] clinically significant difference in weight gain as 1.5 kg, error of 5% and 95% power, estimated as 28 in each group using nMaster 1.0 software.
There were two groups of 28 subjects each. Group I consisted of subjects receiving Tab Amigress 200mg BD orally (amisulpride) and Group II consisted of subjects receiving Tab Elicia 4mg BD orally (blonanserin).
Allocation of the subjects was done by block randomization (Total 7 blocks with 8 subjects each) and it was concealed using sequentially numbered, opaque, sealed envelopes.
Inclusion criteria
a) Both sexes aged between 18-60 years.
b) Subjects with recent onset of psychotic symptoms and those with past history of psychotic symptoms having recurrence of psychotic symptoms currently.
c) Subjects who give informed consent.
Exclusion criteria
a) Subjects with suicidal tendencies.
b) Subjects having diabetes mellitus and abnormal lipid profile.
c) Acute infections or any serious concurrent illness.
d) Presence of organic brain syndrome (delirium, dementia).
e) Presence of alcohol and substance abuse/dependence, epilepsy, mental retardation, pregnancy and lactation.
f) Subjects who are on or who have received other antipsychotics in the last year.
g) Non complying subjects who are unable to give consent for the study.
Methodology
After obtaining clearance from the institutional ethics committee, persons who attended psychiatry department at KR Hospital, attached to Mysore Medical College and Research Institute, Mysore, diagnosed with psychotic disorder according to DSM-IV-TR [14] criteria by the treating psychiatrist, were included in the trial after they met the inclusion and exclusion criteria, after obtaining informed consent. This study was conducted according to the International Conference on Harmonisation - World Health Organization Good Clinical Practice (ICH-GCP) guidelines and the Modified Declaration of Helsinki [15]. Drugs were given free of cost throughout the study. Socio demographic data was collected using a proforma. During the first assessment, before the subject was started on medication, the parameters assessed were body weight, body mass index (BMI) and waist to hip ratio (WHR), which were repeated during the second visit and third visit, which was done at 4 weeks and 8 weeks respectively. Weight was measured using OMRON HN-286 model instrument throughout the study and stretch resistant measuring tape was used for height and WHR measurement.
Statistical Analysis
The data in this study was assessed using the following statistical tests.
• Descriptive statistics: Mean, SD and proportions was used to characterize demographic and clinical data of the sample. Chi-square test was used.
• T-test – independent samples: was used to compare means between the two groups of subjects with regard to weight, BMI and WHR.
• Repeated measure ANOVA: was used to measure the changes of weight, BMI and WHR, during the study (Baseline, 4 weeks and 8 weeks) among the subjects within the same group.
• Repeated measure two-way ANOVA: was used to measure the changes of weight, BMI and WHR, during the study (Baseline, 4 weeks and 8 weeks) between the subjects of the two groups (Amisulpride and blonanserin).
• P-values <0.05 were considered statistically significant.
Using SPSS for Windows (version 14.0)
Results
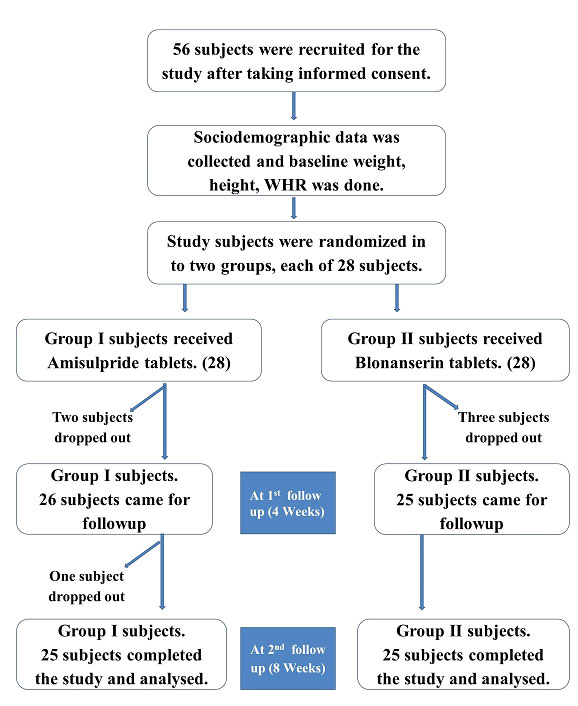
A total of 56 subjects were recruited in the study, 28 subjects in each group. [Table/Fig-1] shows the flow of study participants. In Group I (Amisulpride), 2 subjects were dropped out of the study, during the first follow up and 1 subject dropped out during the second follow up and the reason for drop out was not known. In Group II (Blonanserin), 3 subjects dropped out of the study during the first follow up, 2 subjects because of the EPS, and the reason was not known for 1 subject. Twenty Five subjects in each group completed the study and they were included in the analysis with power of study being reduced to 85%. There was no statistically significant difference in the age and gender distribution, education and occupational status, between the study subjects of the two groups. There was no statistically significant difference in the baseline values of weight, BMI and WHR among the subjects of the two groups. Hence the two groups were comparable [Table/Fig-2].
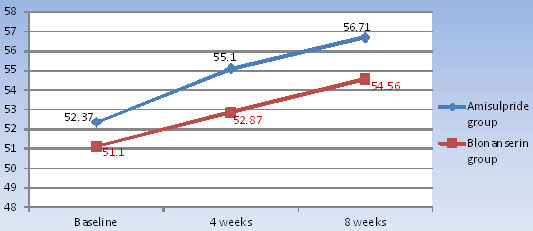
[Table/Fig-3] shows, in amisulpride group, the mean increase in the weight, from baseline to 4 weeks was 2.73 ± 1.20 (5.21%) and from baseline to 8 weeks was 4.34 ± 1.44 (8.28%). In blonanserin group, the mean increase in the weight from baseline to 4 weeks was 1.77 ± 1.35 (3.46%) and from baseline to 8 weeks was 3.46 ± 1.97 (6.75%) [Table/Fig-4]. There was statistically significant (p <0.0001) increase in body weight from baseline to 8 weeks in both amisulpride and blonanserin group, but there was no statistically significant difference seen between the two groups.
In amisulpride group, the mean increase in the BMI from baseline to 4 weeks was 1.04 ± 0.45 and from baseline to 8 weeks was 1.66 ± 0.56. In blonanserin group, the mean increase in the BMI from baseline to 4 weeks was 0.69 ± 0.52 and from baseline to 8 weeks was 1.34 ± 0.77. During the study, there was statistically significant (p <0.0001) increase in BMI, with both the drugs, but there was no difference in the change seen between the two drugs.
In amisulpride group, the mean increase in the WHR from baseline to 4 weeks was 0.017 ± 0.013 and from baseline to 8 weeks was 0.036 ± 0.026. In blonanserin group, the mean increase in the WHR from baseline to 4 weeks was 0.014 ± 0.012 and from baseline to 8 weeks was 0.029 ± 0.020. Statistically significant WHR change was seen with both the drugs, with no difference between them.
Discussion
Weight gain
In the present study, there was statistically significant weight gain associated with amisulpride, which is not consistent with the earlier studies of amisulpride, which suggest that, it was associated with only a slight weight gain. Kotan et al., found that amisulpride is associated with only a slight weight gain of approximately 0.8 kg within 24 weeks [9], Peuskens et al., reported lower risk of weight gain with amisulpride [16], and Leucht et al., stated that mean weight gain with amisulpride (doses above 400 mg/day) is 1.27 kg in 6 months which is not clinically significant [17]. Blonanserin also showed statistically significant weight gain during the study which is not consistent with the earlier studies of blonanserin, which suggested that, it was associated with very less weight gain. Deeks et al., found during long-term treatment with blonanserin in non-comparative trials, patients experienced no significant changes in body weight and fewer than 9% of patients experienced increase in bodyweight [12]. Murasaki et al., and Takahashi et al., also showed the incidence of increased bodyweight was low with blonanserin [18,19].
Even though, amisulpride caused slightly greater weight gain in comparison to blonanserin, the difference in weight gain between the two drugs was not statistically significant (p-value = 0.461). There are no studies comparing the change in weight between blonanserin and amisulpride. Antipsychotic induced weight gain increases the patients’ risk for diabetes mellitus (DM), cardiovascular morbidity and mortality [20]. The underlying mechanisms of weight gain are not clear, and several studies have revealed that antagonistic properties of histamine H1, 5HT2C and muscarinic receptors are involved in antipsychotic-induced weight gain/obesity [1,21,22]. There are reports of paradoxical weight gain associated with amisulpride [23], but the reason for the same could not be ascertained, but could be due it its action on various receptors in the CNS like H1 receptors, and persons of psychosis tend to have poor levels of nutrition, and on starting treatment with antipsychotic drugs, their nutrition will improve and this could be one of the reasons for weight gain as well.
BMI (Body Mass Index)
In the present study, both amisulpride and blonanserin caused statistically significant (p-value <0.0001) increase in BMI during the study period of 8 weeks, which is in contrast with the earlier studies of Kotan et al., and Leucht et al., which showed that amisulpride was not associated with significant changes in BMI [9,17], and the study of Deeks et al., states that blonanserin is not associated with any changes in BMI [12].
WHR (Waist-Hip-Ratio)
WHR is used as a measurement of obesity, and has been shown to be a better predictor of cardiovascular disease than waist circumference and body-mass index alone [24]. In the present study, there was a significant increase in WHR with both amisulpride and blonanserin, and these drugs can increase the cardiovascular disease risk. There are no studies comparing the change in WHR between blonanserin and amisulpride.
Flow chart of the events of this study
Age, Sex distribution, Weight, BMI and WHR among the study subjects
| Demographic data | Group I (Amisulpride) | Group II (Blonanserin) | p-value |
|---|
| Age in years | 18-30 | 13 (52%) | 12 (48%) | 0.777 |
| 31-60 | 12 (48%) | 13 (52%) |
| Total | 25 (100%) | 25 (100%) |
| Mean age ± SD | 31.84 ± 11.18 | 31.56 ± 10.83 |
| Sex | Male | 12 (48%) | 14 (56%) | 0.571 |
| Female | 13 (52%) | 11 (44%) |
| Total | 25 (100%) | 25 (100%) |
| Weight (1st assessment) | 52.37 ± 8.41 | 51.10 ± 9.93 | 0.628 |
| BMI (1st assessment) | 19.96 ± 2.59 | 19.37 ± 3.36 | 0.490 |
| WHR 1st assessment) | 0.856 ± 0.058 | 0.859 ± 0.067 | 0.841 |
BMI= Body Mass Index, WHR= Waist Hip Ratio, ANOVA= Analysis of Variance
Weight, BMI and WHR changes among the study subjects
| Parameters | Baseline (Mean ± SD) | 4 weeks (Mean ± SD) | 8 weeks (Mean ± SD) | Repeated measure ANOVA; p value |
|---|
| Weight (in KG) | Group I (Amisulpride) | 52.37±8.41 | 55.10±8.85 | 56.71±8.86 | <0.0001* |
| Group II (Blonanserin) | 51.10±9.93 | 52.87±9.13 | 54.56±8.72 | <0.0001* |
| Comparison of weight change in group I with group II by using repeated measure 2-way ANOVA; p-value = 0.461 |
| BMI (Body Mass Index) | Group I (Amisulpride | 19.96±2.59 | 20.99±2.72 | 21.62±2.76 | <0.0001* |
| Group II (Blonanserin) | 19.37±3.36 | 20.06±3.14 | 20.71±3.09 | <0.0001* |
| Comparison of BMI change in group I with group II by using repeated measure 2-way ANOVA; p-value = 0.334 |
| WHR (Waist Hip Ratio) | Group I (Amisulpride) | 0.856±0.058 | 0.873±0.056 | 0.892±0.058 | <0.0001* |
| Group II (Blonanserin) | 0.859±0.068 | 0.874±0.062 | 0.888±0.059 | <0.0001* |
| Comparison of WHR change during the study period (8 weeks) in group I with group II by using repeated measure 2-way ANOVA; p-value = 0.994 |
Weight changes among the study subjects
Limitations of the study
• This was an open labeled study, with a small sample size.
• Short duration of follow-up.
• Subjects were not put on a standardized diet and their activity status was not evaluated.
Conclusion
Even though there was no significant difference in the weight gain caused by blonanserin, in comparison with amisulpride, both these drugs individually caused significant weight gain at 8 weeks, which is in contrast with the earlier studies, which needs to be further evaluated.
BMI= Body Mass Index, WHR= Waist Hip Ratio, ANOVA= Analysis of Variance
[1]. JM Meyer, Pharmacotherapy of Psychosis and Mania. In: Brunton L L, Chabner B A, Knollmann B C, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics 2011 12th EditionNew YorkMcGraw-Hill:417-56. [Google Scholar]
[2]. A Gosh, K Chakraborty, SK Mattoo, Newer molecule in the treatment of schizophrenia: A clinical updateIndian Journal of Pharmacology 2011 43(2):105-12. [Google Scholar]
[3]. AJ Lieberman, G Tollefson, M Tolen, PH Dr, AI Green, RE Gur, Comparative efficacy and safety of atypical antipsychotic drugs in first-episode psychosis: A randomized, double blind trial of olanzapine versus haloperidolAmerican Journal of Psychiatry 2003 160(1):1396-404. [Google Scholar]
[4]. M Kannabiran, V Singh, Metabolic syndrome and atypical antipsychotic: A selective literature reviewGerman Journal of Psychiatry 2008 11:111-22. [Google Scholar]
[5]. T Silverstone, G Smith, E Goodall, Prevalence of obesity in patients receiving depot antipsychoticsBr J Psychiatry 1993 162:249-50. [Google Scholar]
[6]. DB Allison, JL Mentore, M Heo, LP Chandler, JC Cappelleri, MC Infante, Antipsychotic-induced weight gain: a comprehensive research synthesisAm J Psychiatry 1999 156(11):1686-96. [Google Scholar]
[7]. MP Curran, CM Perry, Amisulpride: a review of its use in the management of schizophreniaDrugs 2001 61(14):2123-50. [Google Scholar]
[8]. MF Juruena, EP de Sena, IR de Oliveira, Safety and tolerability of antipsychotics: focus on amisulprideDrug Healthc Patient Saf 2010 2:205-11. [Google Scholar]
[9]. Z Kotan, B Ertepe, C Akkaya, E Sarandol, G Ozkaya, A Kirli, Metabolic, endocrinologic and cardiac effects of amisulpride: a 24-week follow-up studyTherapeutic Advances in Psychopharmacology 2011 1(6):189-96. [Google Scholar]
[10]. T Une, S Kurumiya, Pharmacological profile of blonanserinJpn J Clin Psychopharmacol 2007 10(7):1263-72. [Google Scholar]
[11]. M Murasaki, H Nishikawa, T Ishibashin, Dopamine-serotonin antagonist: Receptor binding profile of a novel antipsychotic blonanserinJpn J Clin Psychopharmacol 2008 11(5):845-54. [Google Scholar]
[12]. ED Deeks, GM Keating, Blonanserin: a review of its use in the management of schizophreniaCNS Drugs 2010 24(1):65-84. [Google Scholar]
[13]. C Crisan, C Marginean, L Fodoreanu, D Ponta, The correlation between metabolic effects and antipsychotic therapyRomanian Journal of Psychiatry 2009 1:38-43. [Google Scholar]
[14]. DSM IV criteria for Schizophrenia and Other Psychotic Disorders, Diagnosticand Statistical Manual of Mental disorder4th EditionWashington DCAmerican Psychiatry Association [Google Scholar]
[15]. http://apps.who.int/prequal/info_general/documents/GCP/gcp1.pdf (Accessed on 19/04/2015) [Google Scholar]
[16]. J Peuskens, M De Hert, A Mortimer, for the SOLIANOL Study Group. Metabolic control in patients with schizophrenia treated with amisulpride or olanzapineInt Clin Psychopharmacol 2007 22(1):145-52. [Google Scholar]
[17]. S Leucht, S Wagenpfeil, J Hamann, W Kisslingy, Amisulpride is an “atypical” antipsychotic associated with low weight gain.Psychopharmacology (Berl) 2004 173:112-15. [Google Scholar]
[18]. M Murasaki, Long-term clinical study of blonanserin for schizophrenia: a multicenter open study to determine safety and effectiveness in schizo¬phrenic patients (Kanagawa Region Clinical Psychopharmacology Study Group) Jpn J Clin Psychopharmacol 2007 10(2):2241-57. [Google Scholar]
[19]. S Takahashi, M Suzuki, M Uchiyama, One-year follow-up study of psychotic patients treated with blonanserin: a case seriesAsia Pac Psychiatry 2013 5(3):164-67. [Google Scholar]
[20]. JJ Guo, PE Keck, PK Corey-Lisle, Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: a retrospective, population-based, case-control studyJ Clin Psychiatry 2006 67:1055-61. [Google Scholar]
[21]. SM Stahl, Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications 2008 third EditionNew York, NYCambridge University Press [Google Scholar]
[22]. WK Kroeze, SJ Hufeisen, BA Popadak, SM Renock, S Steinberg, P Ernsberger, H1-Histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugsNeuropsychopharmacology 2003 28:519-26. [Google Scholar]
[23]. GN Papadimitriou, CG Theleritis, DG Dikeos, CJ Psarros, CR Soldatos, Acute weight gain induced by amisulpride monotherapy in a first-episode schizophrenic patientInt Clin Psychopharmacol 2006 21(6):181-84. [Google Scholar]
[24]. B Morkedal, PR Romundstad, LJ Vatten, Informativeness of indices of blood pressure, obesity and serum lipids in relation to ischaemic heart disease mortality: the HUNT-II studyEuropean Journal of Epidemiology 2011 26(6):457-61. [Google Scholar]