Introduction
Squamous cell carcinoma (SCC) accounts for the most common malignancy occurring in the oral cavity, of which tobacco and alcohol are the two well established risk factors [1] with diet, immunodeficiency and viruses being the important co-factors [2].
Recent trends show increasing incidence of Head and Neck Squamous Cell Carcinoma (HNSCC) occurring in non smoking, non alcoholic, young age individuals and the cause has been attributed to the role of Human Papilloma Virus (HPV). The association of HPV with HNSCC was first suggested by Finnish team led by Stina Syrjanen in the year 1983 who found histological and morphological similarities with HPV associated lesions in 40% of the HNSCC cases [3]. According to a review by Kreimer and colleagues, 24% of Oral SCC worldwide showed presence of HPV DNA [4]. However, from the molecular perspective, presence of HPV DNA alone is not sufficient to give a diagnosis of HPV positive HNSCC [5]. The gold standard for classification of HPV caused cancer remains with the expression of HPV oncogenes, E6 and E7 which are required for tumour initiation [6] and maintenance of malignant phenotype [7,8].
Human Papilloma Virus is a non enveloped, double stranded, epitheliotrophic, circular DNA virus belonging to family Papovaviridae. Currently identification of over 81 different subtypes of HPV have been done, and separated on the basis of tropism for cutaneous and mucosal sites. Moreover, depending upon association with malignancy, they are categorized as high, intermediate and low risk [9]. Out of all subtypes, HPV 16 accounts for the most common type seen in HNSCC [10].
Most of the cases of HPV positive HNSCC arise from the oropharyngeal region due to the possibility that this area is more predisposed to epithelial injury due to its location and absence of protective keratin layer which results in easy exposure of the basal cells to the virus [1,10].
HPV genome comprises of double stranded circular DNA carrying early open reading frame (ORF), late ORF and non coding upstream regulatory region between early and late regions [11]. [Table/Fig-1] shows the different parts of HPV genome, namely early and late ORF’s and the upstream regulatory region.
Different parts of HPV genome

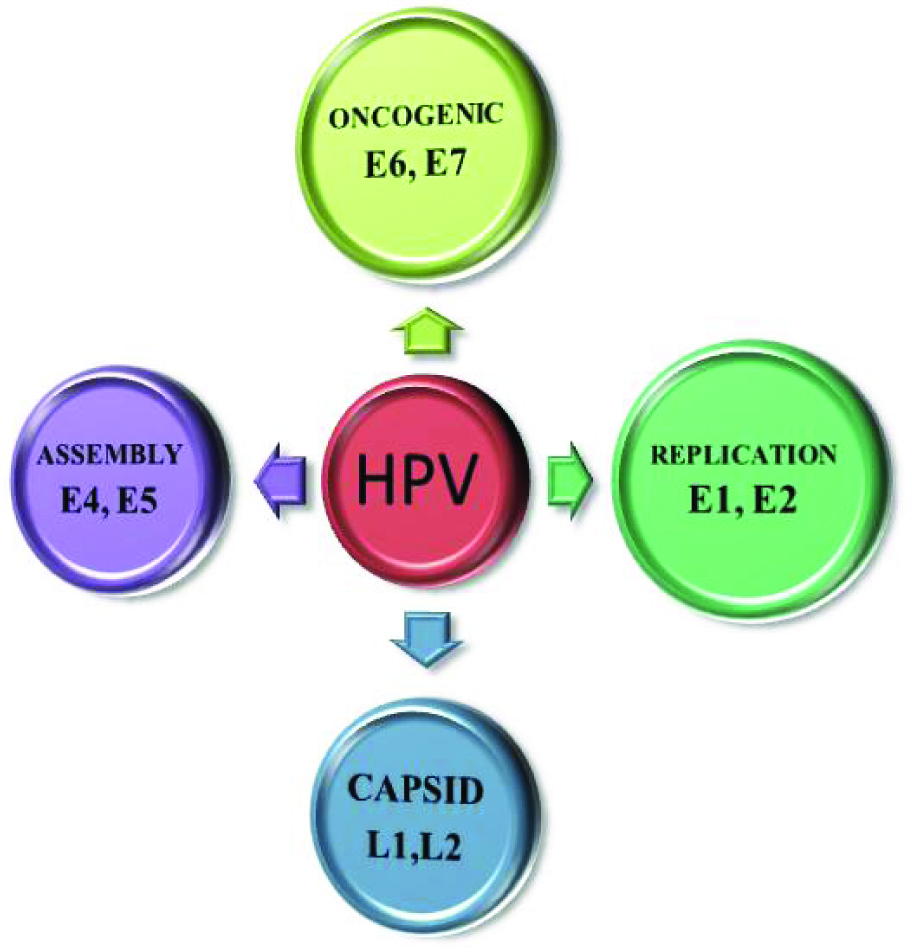
HPV produces different types of proteins which are destined to perform various functions like replication and assembly of virus particles in the superficial layers of epithelium. Few proteins code for the capsid formation and few act as oncoproteins [12]. [Table/Fig-2] gives an overview of different proteins expressed by HPV and their functions.
Proteins expressed by HPV
Entry and Replication of HPV
Sexual and close contact, auto inoculation and mother to fetus transmission are the various modes of acquiring oral HPV infections [13]. Viral entry occurs through micro wounds of the epithelium [14]. The exact receptor and the mechanism for viral entry are not known. Heparin sulphate is thought to assist in the initial attachment of virus particles to the cell, after which the virus enters the cell by endocytosis [15].
HPV infection of epithelial cells results in expression of different viral proteins which produces around 20 to 100 viral DNA per cell in the basal layer and around thousands in the superficial layers. The viral proteins E1 and E2 maintain the number of viral DNA copies throughout the course of infection by regulating viral DNA transcription [16]. In the superficial layers, with viral DNA copies in thousands, the late phase proteins namely E4, E5, L1, L2 are produced. Then the virus particles get assembled by the help of E4 and E5, encapsulated with the help of L1 and L2 proteins making the viral laden cells ready for desquamation. [Table/Fig-3] gives a step wise mechanism of entry, replication and release of HPV.
Flow chart showing entry, replication and release of HPV

Molecular Pathogenesis
HPV oncoproteins E6 and E7 have an important role in increased cell division and mainly function by acting on p53 and Rb genes (tumour suppressor genes, regulates the cell cycle) rendering them inactive which leads to immortalization of cells and hence cancer [17,18].
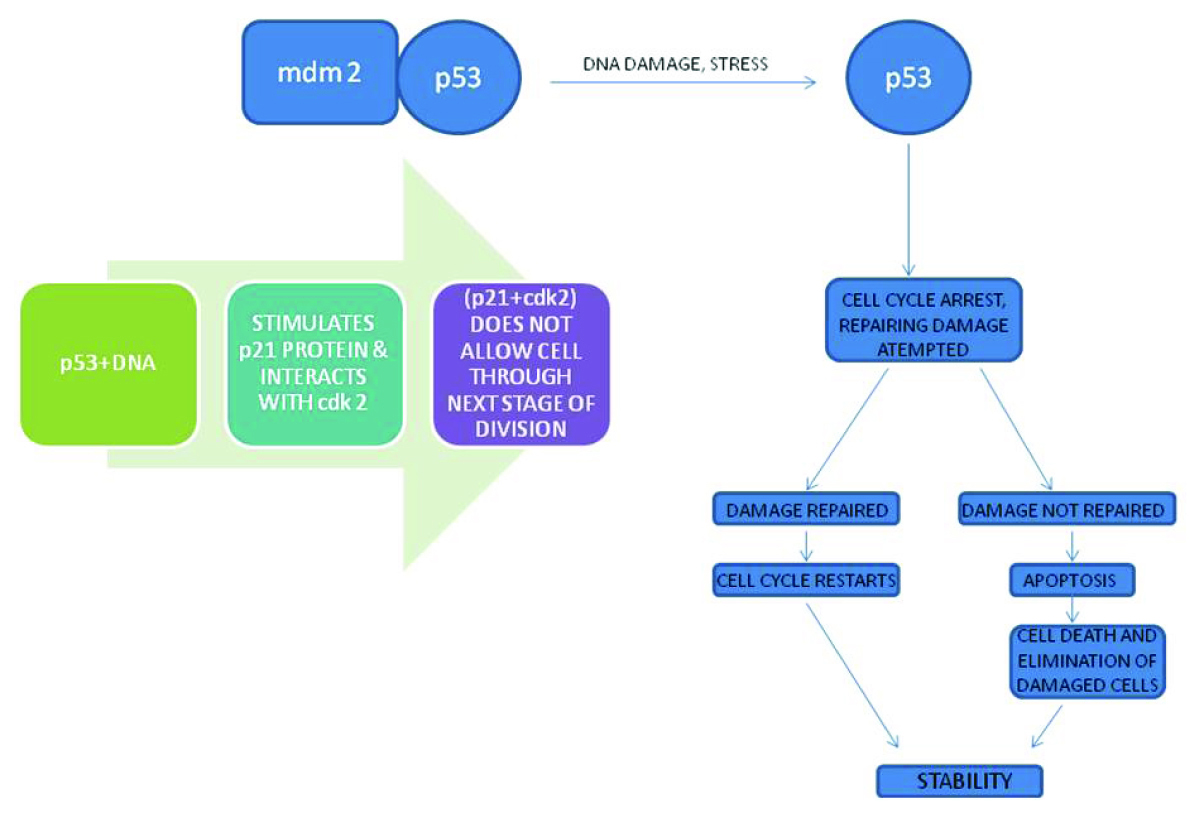
Role of p53 in Normal Cells
p53 (Guardian of Genome), tries to repair the DNA damage and suppresses cell growth by controlling entry into S phase of cell cycle and by reducing DNA synthesis. In normal cells, p53 remains in association with MDM2, a protein which is responsible for its destruction and hence has a short half life of 20 minutes. When the cell is subjected to any stress, p53 undergoes post transcriptional modification and gets detached from MDM2, thus increasing its half life. p53 causes cell cycle arrest and attempts DNA repair. p53 interacts with DNA, and in turn stimulates p21 protein which interacts with cdk 2 and this (p21+cdk 2) complex does not allow the cell through the next stage of division. If the damage is repaired, cell cycle restarts regaining stability. But if p53 is unable to repair the damage, it causes the cell to undergo apoptosis, thus causing elimination of the damaged cells and maintains stability [18].
Action of Oncoprotein E6 on p53
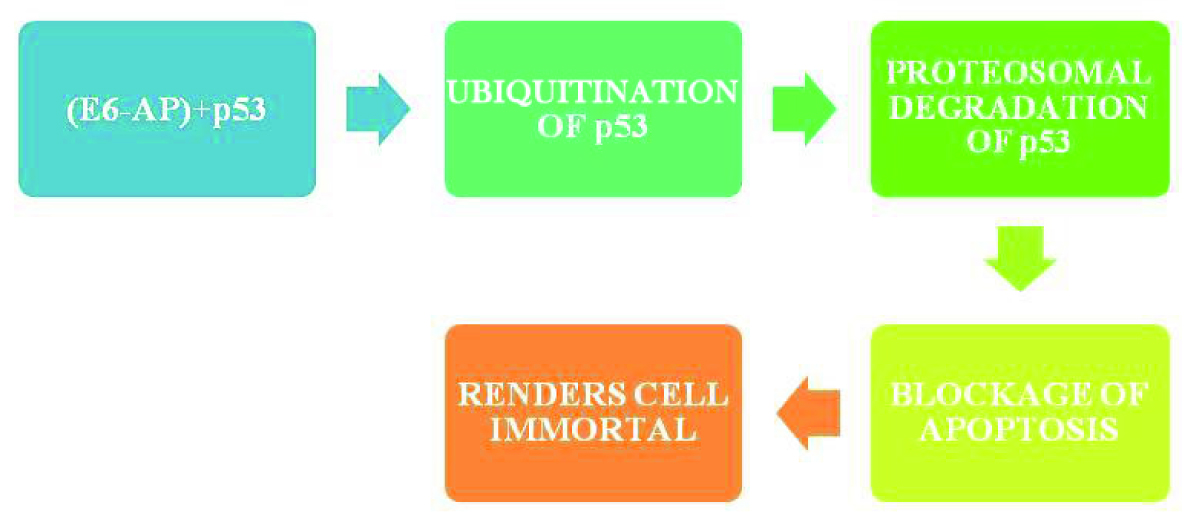
In HPV induced HNSCC, oncoprotein E6 targets p53, Bak, Myc proteins which regulate apoptosis [19]. E6 binds to p53 resulting in its ubiquitination (post translational modification) and proteosomal degradation [16], which blocks apoptosis and promotes uncontrolled division rendering the cell immortal. [Table/Fig-4] depicts the function of p53 in normal cells and [Table/Fig-5] shows the action of oncoprotein E6 on p53.
Function of p53 in normal cells
HPV infected cells - action of oncoprotein E6 on p53
Role of Retinoblastoma (RB) in Normal Cells
Rb is a nuclear phosphoprotein which plays a major role in enforcement of G1, gap between M and S phase of cell cycle. It remains active in a hypophosphorylated form. Promoter such as cyclin E which is functionally dependant on the Elongation Factor E2F, forms a complex with cdk 2 (cyclin E-cdk 2 complex) and initiates DNA replication. In its hypophosphorylated form, Rb represses gene transcription required for transition from G1 to S phase by directly binding to E2F and by binding to the promoter of these genes as a complex with E2F. Rb represses transcription also by remodelling chromatin structure through interaction with proteins such as Brahma-Related Gene 1 (BRG1), Histone deacetylase 1 (HDAC1), human Brahma (hBRM) and Suppressor of Variegation 3-9 Homolog 1 (SUV39H1). In this manner, Rb helps in controlling cell division and hence acts as a tumour suppressor gene [20].
Action of Oncoprotein E7 on Rb
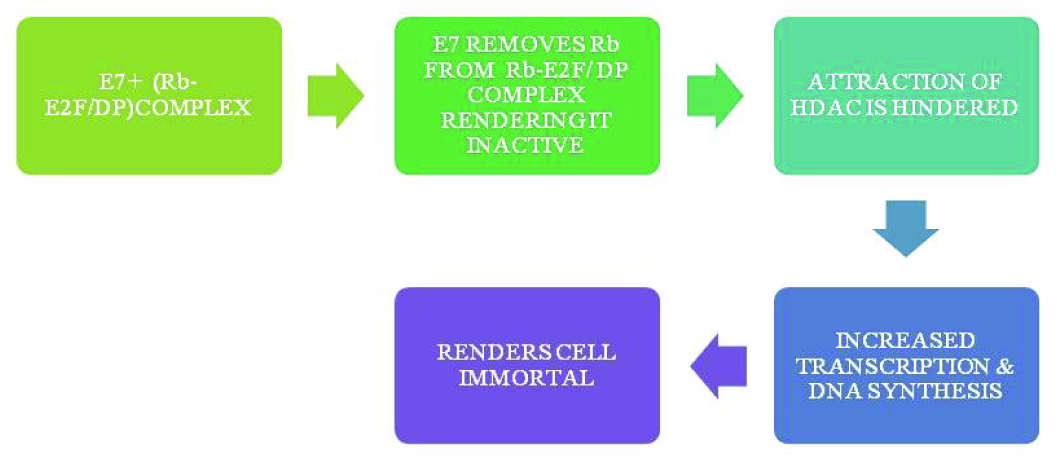
Oncoprotein E7 targets Rb-E2F complex, releasing Rb from the complex and rendering it inactive. Moreover, it also hinders the attraction of HDAC. Both these factors result in increased transcription and increased DNA synthesis, thus rendering the cell immortal [16]. [Table/Fig-6] shows the normal activity of Rb and [Table/Fig-7] shows the action of oncoprotein E7 on Rb.
Function of Rb in Normal cells

HPV infected cells- action of oncoprotein E7 on Rb
HPV Detection Methods
HPV cannot be propagated in tissue culture and thus molecular methods of detection remains a mainstay in detection of HPV positive HNSCC. The current gold standard for establishing a causal role for oncogenic HPV in human tumours remains expression of oncoproteins E6 and E7. According to sensitivity and specificity, detection methods are classified into low, moderate and high sensitivity tests.
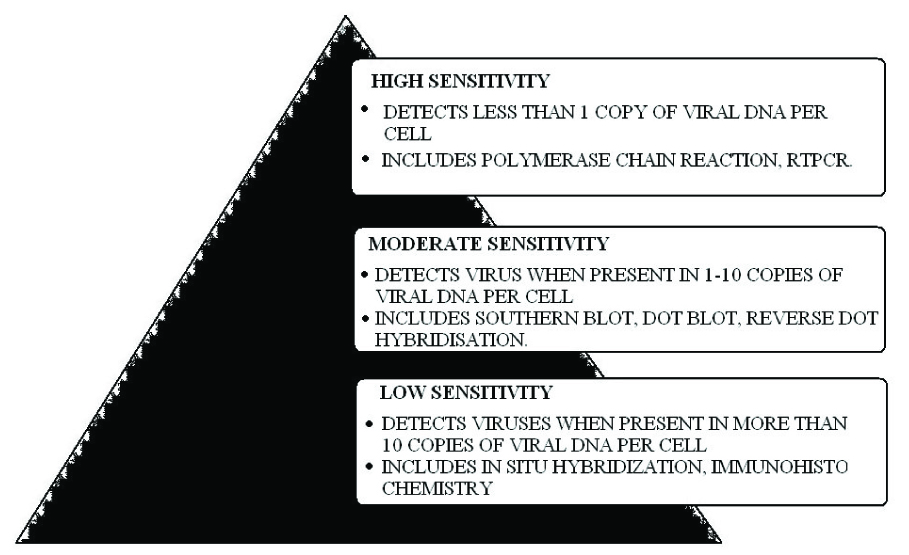
Low sensitivity tests detect viruses when they are present in more than 10 copies per cell. It includes Immunohistochemistry (IHC), In situ hybridization. Moderate sensitivity tests detect viruses when present in 1-10 copies of viral DNA per cell and include methods like Southern Blot, Dot Blot and Reverse Dot Hybridization. High sensitivity tests detect viruses even when less than 1 copy of viral DNA is present per cell. It includes highly specific methods like Polymerase Chain Reaction (PCR) and Reverse Transcriptase PCR (RT-PCR) [21,22]. [Table/Fig-8] gives an overview of these different methods.
Overview of molecular detection methods
Although not pathognomonic of the entity, histopathological features of HPV induced SCC encompasses minor and major criteria, of which minor criteria has limited sensitivity and does not determine the sub-type of HPV.
Presence of Dyskeratosis.
Presence of Atypical immature metaplasia.
Presence of Macrocytes.
Presence of Binucleation.
Presence of classic Koilocytes.
Presence of perinuclear cytoplasmic halos.
Presence of nuclear dysplasia.
Clinical Aspect of HPV Positive Head and Neck Cancers
It is impossible to clinically distinguish between HPV positive and negative Squamous Cell Carcinoma, however the age and personal history of the patient may give a slight indication towards HPV positive cases. For a confirmed diagnosis, the above mentioned investigations can be done.
Recent studies have shown that HPV positive cases showed better response after treatment (includes chemotherapy and chemoradiation), much improved prognosis, survival rate and risk of progression when compared to HPV negative cases [23]. The probable reason for this being absence of field cancerization and the fact that E6 and E7 render p53 and Rb inactive but intact, and removal of E6, E7 could lead to restoration of apoptotic pathway which renders the tumour more sensitive to treatment. On the contrary in HPV negative cases, p53 and Rb get mutated which results in deletion of their activity and presence of field cancerization typically seen in alcohol and tobacco related tumours, hence poorer prognosis [24]. The differences between HPV positive and HPV negative squamous cell carcinoma are enlisted in [Table/Fig-9] [25,26].
Differences between HPV Positive and Negative SCC
| HPV Positive | HPV Negative |
|---|
| Biology and Mutation | p53 inhibited by E6. Rb inhibited by E7 Compensatory increase
in p16 | p53 gets mutated and hence inhibited p16 lost and hence pathways inactive |
| Histopathology | Poorly differentiated or basaloid SCC, presence of koilocytes | Well, moderately or poorly differentiated SCC with kertain pearls |
| Age | More commonly seen in younger individuals | More prevalent in older individuals |
| Risk Factors | Sexual factors | Tobacco, alcohol, diet, immunodeficiency |
| Location | Oropharyngeal region | Floor of month, lateral border of tongue |
| Prognosis | Good | Poor |
Conclusion
With the increasing incidence of HPV OSCC cases, it is important to distinguish between HPV positive and HPV negative OSCC due to the difference in prognosis. Hence, early detection would be the mainstay in treatment and for getting a better prognosis and survival rate. The role of HPV vaccines still remains unclear in prevention of HNSCC. Further studies are necessary in this field.
[1]. Rajendran R, Benign and Malignant tumors of the Oral cavity. In, Rajendran R, Sivapathasundaram B (Ed)Shafer’s Textbook of Oral Pathology 2009 6th editionElsevier:101-04. [Google Scholar]
[2]. Mehrotra R, Yadav S, Oral squmaous cell carcinomaIndian J Cancer 2006 43(2):60-66. [Google Scholar]
[3]. Syrjanen K, Syrjanen J, Lamberg M, Pyrhonen S, Morphological and immunohistochemical evidence suggesting Human Papilloma Virus (HPV) involvement in Oral Squamous Cell CarcinomaInt J Oral Surg 1983 12(6):418-24. [Google Scholar]
[4]. Kreimer AR, Clifford GM, Boyle P, Franceschi S, Human Papillomavirus Types in Head and Neck Squamous Cell Carcinomas Worldwide: A Systematic ReviewCancer Epidemiol Biomarkers Prev 2005 14(2):467-75. [Google Scholar]
[5]. Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, Low etiologic fraction for high risk human papillomavirus in oral cavity squamous cell carcinomasOral oncol 2013 49(1):1-8. [Google Scholar]
[6]. Park NH, Min BM, Li SL, Huang MZ, Cherick HM, Doniger J, Immortalization of normal human oral keratinocytes with type 16 human papillomavirusCarcinogenesis 1991 12(9):1627-31. [Google Scholar]
[7]. Ramplias T, Sasaki C, Weinberger P, Psyrri A, E6 and E7 silencing and transformed phenotype of human papillomavirus type 16- positive oropharyngeal cancer cellsJ Natl Cancer Inst 2009 101(6):412-23. [Google Scholar]
[8]. Tang AL, Hauff SJ, Owen JH, Graham MP, Czerwinski MJ, Park JJ, UM-SCC-104: a new human papillomavirus-16-positive cancer stem cell containing head and neck squamous cell carcinoma cell lineHead Neck 2012 34(10):1480-91. [Google Scholar]
[9]. Hausen HZ, Papilloma Viruses and cancer: From basic studies to clinical applicationNat Rev cancer 2002 2(5):342-50. [Google Scholar]
[10]. Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT, Human Papillomavirus type 16 and squamous cell carcinoma of head and neckClin Cancer Res 2002 8(10):3187-92. [Google Scholar]
[11]. Bonnez W, PapillomavirusIn Clinical virology 2002 2nd editionWashington D.C.ASM Press:557-596.Edited by: Richman DD, Whitley RJ, Hayden FG. [Google Scholar]
[12]. Morshed K, Gruszka DP, Szymanski M, Dacewicz MP, Human Papilloma Virus – Structure, Epidemiology, PathogenesisOtolaryngologia Polska 2014 68(5):213-19. [Google Scholar]
[13]. Syrjanen S, Puranen M, Human papilloma virus infections in children: the potential role of maternal transmissionCrit Rev Oral Biol Med 2000 11(2):259-74. [Google Scholar]
[14]. Suvarna M, Lalith Prakash Chandra K, Chandra Sekhar P, Reddy BVR, HPV Induced Head and Neck Cancer- An Insight into the Molecular PathogenesisJ Orofac Sci 2010 2(3):71-6. [Google Scholar]
[15]. Doorbar J, The papillomavirus life cycleJ Clin Virol 2005 32(suppl 1):S7-S15. [Google Scholar]
[16]. Hausen HZ, Papillomaviruses Causing Cancer: Evasion From Host-Cell Control in Early Events in CarcinogenesisJ Natl Cancer Inst 2000 92(9):690-98. [Google Scholar]
[17]. Miller CS, Pleiotrophic mechanisms of virus survival and persistenceOral Surg Oral Med Oral Pathol Oral Radiol Endod 2005 100(2 Suppl):S27-36. [Google Scholar]
[18]. Stricker TP, Kumar V, Neoplasia. Robbins & Cotran Pathologic Basis of Diseases8th Edition:290-292. [Google Scholar]
[19]. Pillai MR, Nair MK, Development of a Condemned Mucosa Syndrome and Pathogenesis of Human Papillomavirus-Associated Upper Aerodigestive Tract and Uterine Cervical TumoursExp Mol Pathol 2000 69(3):233-41. [Google Scholar]
[20]. Giacinti C, Giordano A, RB and cell cycle progressionOncogene 2006 25(38):5220-27. [Google Scholar]
[21]. Lancellotti CLP, Levi JE, Silva MALG, Schwarzschild M, Nicolau SM, Diagnóstico laboratorial. In: Carvalho JJM, Oyakawa NI Consenso Brasileiro do HPV 2000 41a ediçãoSão PauloBG Cultural:45-60. [Google Scholar]
[22]. Zahm DM, Nindl I, Schneider A, Princípios gerais do diagnóstico: detecção do papilomavírus humano. In Gross GE, Barrasso RInfecção por papilomavírus humano: Atlas clínico de HPV 1999 2o edPorto AlegreEditora Artes Médicas:21-45. [Google Scholar]
[23]. Miller CS, White DK, Ky L, Human papillomavirus in oral mucosa, premalignant conditions, and squamous cell carcinoma: A retrospective review of the literatureOral Sug Oral Med Oral Pathol Oral Radiol Endod 1996 82(1):57-68. [Google Scholar]
[24]. Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trialJ Natl Cancer Inst 2008 100(4):261-69. [Google Scholar]
[25]. Campisi G, Giovannelli L, Controversies surrounding human papilloma virus infection, head and neck vs oral cancer, implications for prophylaxis and treatmentHead Neck Oncol 2009 1(8) [Google Scholar]
[26]. Castro TP, Bussoloti Filho I, Prevalence of human papilloma virus in oral cavity and oropharynxBraz J Otorhinolaryngol 2006 72(2):272-82. [Google Scholar]