Giant Platelets in Platelet Donors – A Blessing in Disguise?
Asitava Deb Roy1, Nabajyoti Choudhury2, Deepanjan Ray3
1 Assistant Professor, Department of Pathology and In Charge Transfusion Medicine, IQ City Medical College, Durgapur, West Bengal, India.
2 Senior Consultant and HOD, Department of Transfusion Medicine, Fortis Memorial Research Institute, Gurgaon, Haryana, India.
3 Assistant Professor, Department of Community Medicine, IQ City Medical College, Durgapur, West Bengal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Asitava Deb Roy, Assistant Professor, Department of Pathology and In Charge Transfusion Medicine, IQ City Medical College and Narayana Multispeciality Hospital, Sovapur, Bijra Road, Jaymua, Durgapur-713213, West Bengal, India.
E-mail: asitavadr@gmail.com
Introduction
Inherited thrombocytopenias, including inherited giant platelet disorders (IGPD) are relatively rare, but their prevalence is probably underestimated. Harris platelet syndrome, the most common IGPD reported from Indian subcontinent, mostly from eastern part, is characterised by a low platelet count, high mean platelet volume (MPV) and absence of bleeding.
Aim
A short study was conducted to assess the prevalence of giant platelets in voluntary donors of single donor platelets (SDP) and analyse the effect of transfusion of such SDPs in patients.
Materials and Methods
Voluntary donors of SDPs were screened as per standard guidelines prior to the procedure. A complete blood count (including MPV) along with a peripheral smear was done. A total of 45 donors were screened for plateletpheresis. Following plateletpheresis from these donors, a platelet count from the collection bag was done after one hour. The SDP was transfused as a single unit or divided into two and transfused to the same patient at two different occasions, as per clinical need. Platelet counts on pateints were done after one hour and the platelet recovery was noted.
Results
Out of the 45 donors who were screened, 30 (66.67%) were found to have giant platelets. It was observed that the pre procedure platelet counts in donors having giant platelets were relatively low (1.5 -1.7 lacs) and so also the platelet yield (2.7-3x1011) compared to donors who did not, but the post transfusion platelet recovery was greater.
Conclusion
Since presence of giant platelets has been seen to be common in the Eastern part of India, a peripheral smear examination should always be considered during screening of plateletpheresis donors to avoid rejecting donors with giant platelets whose platelet counts are given falsely low by autoanalysers.
Introduction
The role of apheresis in the management of patients suffering from cancer has always been significant in this era of component therapy. Apheresis is a procedure where blood is collected from a donor, separated into components, one (or more) of the components is retained and the remaining constituents are recombined and returned to the individual. This procedure, when applied to extract platelets from a donor, is called plateletpheresis and the platelet unit derived from such procedure is called a single donor platelet (SDP) [1].
This study was conducted in a newly built tertiary care cancer centre in Eastern India where numerous SDP requisitions were generated every day. The usual indications for such requisitions were mainly stem cell transplants and chemotherapy related thrombocytopenia. These patients were usually from overseas countries and had no donors available with them for the plateletpheresis procedure. Therefore, we had to take the responsibility of motivating and recruiting donors for plateletpheresis. Donor recruitment for plateletpheresis to meet the huge demand from the clinicians was not an easy task to achieve. However, constant counseling of patient’s relatives and friends and motivation of the hospital staff helped us to achieve the goal [2].
One major observation during screening of platelet donors was that most patients did not meet the eligibility criteria for pre-donation platelet count (more than equal to 1.5 lacs/cumm) and majority of these patients had presence of giant platelets in them. But this was an incidental finding and the donors did not have any symptoms related to low platelet count. These donors, however, constituted a huge donor pool and rejection of these donors because of failure to meet the cut-off for pre donation platelet count led to a loss of an otherwise healthy donor pool.
Inherited thrombocytopenias, including inherited giant platelet disorders (IGPD) are relatively rare, but their prevalence is probably underestimated [3]. Harris platelet syndrome, the most common IGPD reported from Indian subcontinent, mostly from eastern part of India, is characterised by a low platelet count, high mean platelet volume (MPV) and absence of bleeding [4,5].
Since the observation was quite frequent, a short study was conducted to assess the prevalence of giant platelets in voluntary donors of single donor platelets (SDP) and analyse the effect of transfusion of such SDPs in patients.
The main aim and objective of this study were to estimate the prevalence of giant platelets in platelet donors, to study the platelet recovery in patients transfused with SDPs and further to compare the platelet recovery in patients transfused with SDP from donors with giant platelets with that in patients transfused with SDP from donors who do not.
Materials and Methods
Voluntary donors of SDPs were screened as per standard guidelines prior to the procedure. A complete blood count (including MPV) along with a peripheral blood smear, to assess the platelet morphology, was done. The study was conducted in Tata Medical Centre, Kolkata, India. A total of 45 donors were screened for plateletpheresis. Following plateletpheresis from these donors, a platelet count from the collection bag was done after 1 hour to estimate the platelet yield. The SDP was transfused as a single unit to the patient, as per clinical need. Platelet counts on patients were done after one hour and the platelet recovery was calculated according to the formula [6].
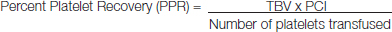
TBV = Total blood volume (75ml/kg for adults)
PCI = Platelet count increment (Platelet count after transfusion – Platelet count before transfusion)
Number of platelets transfused = Platelet yield (estimated from collection bag).
Results
Out of the 45 donors who were screened, 30 (66.67%) were found to have giant platelets. [Table/Fig-1] shows that the pre procedure platelet counts in donors having giant platelets (peripheral smear showing giant platelets in [Table/Fig-2]; platelet histogram showing evidence of presence of giant platelets in [Table/Fig-3]) were relatively low (1.5 -1.8 lacs/cumm) and so also the platelet yield (2.6-3.2x1011) compared to donors who did not, but the post transfusion platelet recovery was greater (44.4-99.29%); mean being 68.1 (SD = 13.39). In donors who did not have giant platelets, the platelet counts were in the range of 1.5 – 3.6 lacs/cumm, platelet yield was 3.1-3.8x1011 and platelet recovery was 25.54-52.5%; mean being 40.79 (SD = 7.36) [Table/Fig-4]. Data is normally distributed as Shapiro-Wilk’s test is not significant (p-value >0.05). Applying independent sample t test, it is seen that there is significant difference in platelet recovery (p-value<0.05) in both the groups (donors with giant platelets versus donors with no giant platelets).
Pre procedure platelet counts in donors having giant platelets and the corresponding PPR data
| DONOR NUMBER | DONOR PLATELET COUNT (lakhs/mm3) | PRODUCT QC COUNT | PRE-TRANSFUSION PLATELET COUNT | POST-TRANSFUSION PLATELET COUNT (AFTER 1HR) | PERCENT PLATELET RECOVERY (PPR) |
|---|
| 1 | 1.50 | 2.9X1011 | 3,000 | 41,000 | 64.125 |
| 2 | 1.65 | 2.8X1011 | 24,000 | 27,000 | 44.4 |
| 3 | 1.80 | 3.1X1011 | 9,000 | 30,000 | 48.75 |
| 4 | 1.59 | 3.1X1011 | 13,000 | 70,000 | 99.29 |
| 5 | 1.65 | 3X1011 | 4,000 | 42,000 | 73.88 |
| 6 | 1.50 | 3.2X1011 | 11,000 | 44,000 | 68.02 |
| 7 | 1.50 | 3.1x1011 | 2,000 | 4,000 | 70.9 |
| 8 | 1.67 | 2.7X1011 | 4,000 | 45,000 | 77.44 |
| 9 | 1.50 | 2.8X1011 | 15,000 | 54,000 | 67.9 |
| 10 | 1.55 | 2.6X1011 | 15,000 | 53,000 | 91.97 |
| 11 | 1.65 | 3X1011 | 13,000 | 54,000 | 71.75 |
| 12 | 1.80 | 3.1X1011 | 11,000 | 44,000 | 67.33 |
| 13 | 1.50 | 3.1X1011 | 7,800 | 48,300 | 68.58 |
| 14 | 1.76 | 3X1011 | 6,000 | 39,000 | 59.4 |
| 15 | 1.66 | 3.2X1011 | 7,500 | 42,000 | 57.41 |
| 16 | 1.78 | 3.1X1011 | 5,500 | 50,000 | 77.51 |
| 17 | 1.69 | 3X1011 | 9,800 | 60,000 | 86.59 |
| 18 | 1.50 | 2.9X1011 | 3,000 | 50,000 | 82.65 |
| 19 | 1.54 | 3X1011 | 8,000 | 38,000 | 56.25 |
| 20 | 1.75 | 3.2X1011 | 14,000 | 53,000 | 63.07 |
| 21 | 1.64 | 3.1X1011 | 24,000 | 27,000 | 44.4 |
| 22 | 1.80 | 2.9X1011 | 9,000 | 30,000 | 48.75 |
| 23 | 1.78 | 3X1011 | 6,000 | 39,000 | 57.44 |
| 24 | 1.75 | 3.2X1011 | 7,500 | 42,000 | 57.41 |
| 25 | 1.67 | 2.7X1011 | 4,000 | 45,000 | 76.44 |
| 26 | 1.50 | 2.8X1011 | 15,000 | 54,000 | 66.9 |
| 27 | 1.55 | 3X1011 | 15,000 | 53,000 | 81.97 |
| 28 | 1.50 | 3.1X1011 | 11,000 | 44,000 | 67.23 |
| 29 | 1.50 | 3.1x1011 | 2,000 | 4,000 | 71.92 |
| 30 | 1.73 | 3X1011 | 4,000 | 45,000 | 75.44 |
Peripheral blood smear of a blood donor showing giant platelets (Leishman, 1000x)

Platelet histogram of a blood donor showing presence of giant platelets

Pre procedure platelet counts in donors without giant platelets and the corresponding PPR data
| DONOR NUMBER | DONOR PLATELET COUNT (lakhs/mm3) | PRODUCT QC COUNT | PRE-TRANSFUSION PLATELET COUNT | POST-TRANSFUSION PLATELET COUNT (AFTER 1HR) | PERCENT PLATELET RECOVERY (PPR) |
|---|
| 1 | 2.37 | 3.4X1011 | 10,000 | 50,000 | 49.3 |
| 2 | 2.40 | 3.7X1011 | 28,000 | 46,000 | 25.54 |
| 3 | 1.97 | 3.6X1011 | 30,000 | 60,000 | 45 |
| 4 | 1.74 | 3.6X1011 | 30,000 | 62,000 | 46.66 |
| 5 | 1.5 | 3.8X1011 | 5,600 | 27,000 | 30.69 |
| 6 | 1.87 | 3.1X1011 | 23,000 | 47,000 | 37.16 |
| 7 | 1.98 | 3.2X1011 | 4,000 | 29,000 | 38.67 |
| 8 | 2.45 | 3.2x1011 | 6,000 | 30,000 | 38.81 |
| 9 | 2.67 | 3.5x1011 | 8,500 | 34,000 | 34.42 |
| 10 | 2.75 | 3.2x1011 | 10,000 | 38,000 | 45.93 |
| 11 | 3.05 | 3.1x1011 | 9000 | 40,000 | 49.92 |
| 12 | 3.6 | 3.4x1011 | 12,000 | 47,000 | 52.5 |
| 13 | 1.65 | 3.1x1011 | 18,000 | 42,000 | 37.18 |
| 14 | 2.67 | 3.2x1011 | 5,000 | 31,000 | 40.21 |
| 15 | 2.87 | 3.2x1011 | 6,500 | 34,000 | 39.96 |
Therefore, it was observed that although the pre-donation platelet count and platelet yield in donors were on the lower range in donors who had giant platelets in them, the platelet recovery was greater compared to that from donors who did not have giant platelets.
Discussion
Of all the inherited thrombocytopenias, Harris platelet syndrome (HPS) is the most common [4]. In 2002, this syndrome was called "Asymptomatic constitutional macro thrombocytopenia" (ACMT) [3]. In 2005, this entity was renamed as Harris platelet syndrome to avoid confusion between ACMT and congenital amegakaryocytic thrombocytopenia (CAMT) [4,5]. HPS was identified among healthy blood donors in the north-eastern part of the Indian subcontinent, characterized by absent bleeding symptoms, mild to severe thrombocytopenia (platelets rarely <50 X 109/L)with giant platelets (Mean platelet volume 10fL) and normal platelet aggregation studies with absent MYH9 mutation [4]. Authors have found a statistically higher MPV, RDW and a lower platelet count and platelet biomass in the blood donors with HPS [3]. The present day diagnosis of HPS is based on ascertaining the ethnicity of the patient, as well as assessing for conditions causing acquired thrombocytopenias, and also excluding the known inherited giant platelet disorders (IGPD) and other congenital thrombocytopenias. It is extremely important to recognize and diagnose Harris platelet syndrome, as almost one third the population of certain parts of Indian subcontinent is affected [5,7].
Earlier studies have shown that larger platelets, as compared with smaller platelets, are more active enzymatically and metabolically and have a higher potential thrombotic ability [8]. It has also been shown that platelet size, when measured as mean platelet volume (MPV), is a marker of platelet function and is positively associated with indicators of platelet activity [8]. However, we currently lack understanding of the predictive accuracy of high MPV in SDP donors for post transfusion platelet recovery in patients. The index study shows that the platelet recovery was greater when patients were transfused with platelet units containing giant platelets. This could be an indicator that giant platelets are functionally more active than normal platelets. However, function of giant platelets need to be assessed by performing platelet function tests to establish this correlation. Future research works based on platelet function with a larger sample size are needed to clarify this issue.
Conclusion
It is recommened that a peripheral smear examination should always be done to verify the platelet counts given by auto-analyser, because in case of presence of giant platelets in donors the auto-analyser may give a false low count and relying on machine counts solely may lead to rejection of donors who actually might be having a platelet count >1.5 lacs per cumm. Therefore, in the Eastern part of India, a peripheral smear examination or platelet count in Neubauer’s chamber should be made an essential component for screening plateletpheresis donors. As the study noted that platelet recovery in patients was greater with transfusion of platelet units containing giant platelets, it might be suggested that plateletpheresis donors with platelet count lower than the eligibility cut-off of 1.5 lacs per cumm, but with presence of giant platelets may be accepted as SDP donors in the Eastern part of India. This, if possible, would add to the precious apheresis donor pool and contribute to the easier management of patients who are in dire need of platelets.
[1]. Francis RR, Apheresis, Chapter 17In: Modern Blood Banking and Transfusion Practices. Denise M. Harmening 1999 4th editionPhiladelphiaF A Davis Company:363 [Google Scholar]
[2]. Deb Roy A, Bagchi IR, Choudhury N, Need for Motivation of Plateletpheresis Donors: A Tertiary Care Cancer Center Experience from Eastern IndiaAnn Med Health Sci Res 2014 4(Suppl 1):S63-64. [Google Scholar]
[3]. Naina HV, Nair SC, Daniel D, George B, Chandy M, Asymptomatic constitutional macrothrombocytopenia among West Bengal blood donorsAm J Med 2002 112(9):742-43. [Google Scholar]
[4]. Naina HV, Nair SC, Harris S, Woodfield G, Rees MI, Harris syndrome - a geographic perspectiveJ Thromb Haemost 2005 3(11):2581-82. [Google Scholar]
[5]. Naina HV, Harris S, Platelet and red blood cell indices in Harris platelet syndromePlatelets 2010 21(4):303-06. [Google Scholar]
[6]. American Association of Blood BanksTechnical Manual 2014 18th edMaryland, USAAABB:361-83. [Google Scholar]
[7]. Naina HV, Harris S, Harris platelet syndrome--underdiagnosed and unrecognizedArch Pathol Lab Med 2008 132(10):1546 [Google Scholar]
[8]. Gohda F, Uchiumi H, Handa H, Identification of inherited macrothrom-bocytopenias based on mean platelet volume among patients diagnosed with idiopathic thrombocytopeniaThromb Res 2007 119(6):741-46. [Google Scholar]