The physiological events in response to infections during the early intrauterine development of an individual determine the vulnerability of immune system of the individual [1]. Untreated gestational infections could cause adverse effects on fetal development and serious postnatal consequences on brain and behaviour, that includes cerebral palsy, schizophrenia, non-genetic form of autism, mental retardation, etc [2–7]. Furthermore, alterations in the offspring immune system may hamper its coordination with nervous and endocrine systems, and leaves the offspring more susceptible to various diseases in the later part of the life [1].
Evidences from animal models of prenatal immune activation using Lipopolysaccharide (LPS) induced infections have clearly delineated the cellular mechanisms and cognitive abnormalities that result from gestational infection induced inflammations [8]. LPS is an integral cell wall component of gram-negative bacteria. Prenatal administration of LPS [9–11] leads to induction of pro inflammatory cytokines and microglia activation in maternal and fetal compartments [12]. These factors, along with elevated TNF-alpha and IL6, could impair the fetal brain development [13,14].
Studies on animal models have reported the enhancing effect of physical exercises on adult neurogenesis and neurogenesis-dependent behavioural tasks [15–18]. However, no such study has analysed their beneficial effect during the early growth spurt period.The objective of the present study was twofold. To investigate whether prenatal exposure to LPS induced inflammation would impair the cognition of an individual and to determine the role of physical exercise, especially in growth spurt period i.e. early postnatal life, in mitigating such cognitive deficits.
Materials and Methods
Adult female Wistar rats (n=16) aged 3-months (200-250g) were used. To induce pregnancy of known gestational days, female rats were caged with adult male rats overnight (2 female rats: 1 male rat). The vaginal smear was examined within 12 hours after mating. The pregnancy was confirmed by the presence of sperms in the smear and was considered as day ‘0’ of pregnancy. Two pregnant female rats were housed together with proper label indicating the day of conception.
The pregnant rats were randomly assigned into a control (n=6) and LPS group (n= 10). LPS (0.5mg/kg, E.coli serotype 0111: B4 Sigma-Aldrich) was injected intraperitoneally (i.p) to the pregnant rats of LPS group, from gestation day 14 till delivery on alternate days. Sterile saline (0.5ml) was injected to the controls.
Following parturition, all the offspring were raised by their biological mothers till the 21st day (weaning) and the litters were culled to six pups. Cross fostering per se alters the behavioural response to LPS hence cross fostering was not allowed [19]. Male pups were retained for the experiment. The animals were housed under controlled conditions of temperature (22 ± 2°C), humidity (50 ± 5%) and a 12-h light/dark cycle, in sanitized polypropylene cages containing sterile paddy husk as bedding, with water and food available ad libitum. All animal experiments were conducted at the same time of day (9:00 - 12:00 am) in Central Animal Research Facility of Manipal University. Total duration of the study was around 3 months that includes pregnancy duration and following parturition offspring were allowed to reach the age of postnatal day (PND) 70. The animals were maintained according to the prescribed guidelines of the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), Government of India. The experiments were approved by the Institutional Animal Ethical Committee (IEAC) of Manipal University.
Physical Exercises and behavioural testing: In addition to the control group of pups (n=6), the pups born to the LPS treated mothers were randomly assigned further into two sub groups i) LPS (n=6) and ii) LPS+Exercise (LPS+Ex) (n=6). The LPS+Ex group was subjected to running exercise in a treadmill, 15 mins/day in 5 sessions of 3 minutes each (6days/week) from PNDs 30 to 60, with approximately 15-20 min intersession interval. The treadmill consisted of motor-driven, 5-lane parallel runways maintained at 0o inclination and running speed was set at 10.2 meters/min (IIITC Life Science, CA, USA. Model 805, Series 800). At the end of each session, the rats were returned to their home cages.
Morris Water Maze test: The Morris Water Maze (MWM), a standard hippocampal-dependent task, was used to assess the spatial learning and memory abilities between PNDs 61 to 67. The water maze consists of a circular pool of 1.5 meters in diameter, divided conceptually into 4 quadrants filled with water (18-23°C to a depth of about 40cm). A hidden escape platform (4″x 4″ size) was submerged in one of the quadrants, the target quadrant, approximately 1cm below the surface of the water. Powdered milk was added to the pool before the experiment to render the water opaque [20]. Permanently positioned distinctive visible cues were maintained for facilitating the spatial orientation of the animal.
The rats were trained in 10 sessions on five consecutive days [21]. Each session consisted of four trials. In each trial time taken to reach the hidden platform was recorded. If the rat did not find the platform within 120 seconds, it was guided gently to the platform and left on it for 20 seconds [14]. Twenty-four hours after the last session, the rats were subjected to memory retention test (probe test) with one session of 60 seconds duration, where the escape platform was removed. The time taken to reach the target quadrant in the 5 days training period and the time spent in the target quadrant during memory retention test were observed visually and timed with stopwatches. Greater latency to reach the target quadrant and less time spent in the target quadrant suggests memory impairment. All the data were collected, processed and analysed by the individual blind to experiments. Briefly, the rats were subjected to 5-day learning and memory training sessions followed by a memory retention test on the 6th day. In order to reduce the excitement of strange environment, all the animals were subjected for a trial on a day before the actual training sessions.
Statistical Analysis
The data was expressed as the mean ± SEM and analysed using SPSS 16.0 (IBM). Comparisons were performed using one-way Analysis Of Variance (ANOVA) followed by Tukey’s post-hoc test. A value of p<0.05 was considered as statistically significant.
Results
Overall escape latency analysis: The overall escape latencies were significant between groups during day1 to 5 of training sessions in MWM (day 1, ANOVA F (2, 17) = 6.060, p <0.05; day 2, ANOVA F (2, 17) = 11.520, p <0.001; day 3, ANOVA F (2, 17) = , p <0.001; day 4, ANOVA F (2, 17) = , p <0.001; day 5, ANOVA F (2, 17) =, p <0.001) [Table/Fig-1]. Post-hoc test revealed there were no significant differences between control and LPS+Ex groups observed during days 1, 2, 3 and 5 of the training period. However, on the day 4 of training, the LPS+Ex group had significant longer latency than the control group (p<0.05).
Mean escape latencies to find the platform across training days in the Morris water Maze performance (mean ± S.E.M.). Subjects are classified by treatment condition (circular symbols - prenatally exposed to sterile saline, square symbols- prenatally exposed to LPS and triangular symbols- prenatally exposed to LPS and subjected to running exercise from PNDs 30 to 60). Symbols denote group means that represent average scores across the eight trials per test day ± S.E.M’s. Asterisk * and *** indicates difference between groups at the p<0.05 and p<0.001 level, respectively
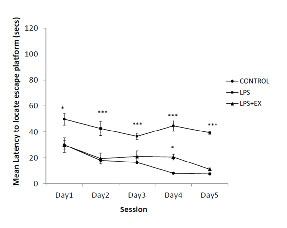
Spatial learning and memory performance in offspring exposed to prenatal inflammation during gestation.
During training sessions of MWM (day 1-5), the offspring in the LPS group displayed significant longer escape latencies to reach hidden platform i.e. longer duration to reach the escape platform, as compared to the age-matched control and LPS+Ex groups [Table/Fig-1]. They also showed notable improvement during first three days of training phase, however, there was a decrement in the performance where the animals displayed longest latency to reach platform, during the day 4 and 5 of training periods and the reason for this performance decrement was unclear. Also, the LPS group had spent significantly lesser duration, than the control and LPS+Ex group, in the target quadrant in search of hidden platform [Table/Fig-2] in the memory retention test (ANOVA F (2,17) =30.251, p<0.001).
Effect of prenatal LPS/saline exposure as well as postnatal running exercise on Morris water Maze performance. The time spent in the target quadrant in search of platform (with platform removed) in Morris water Maze performance in the memory retention test. Results are expressed as Mean± S.E.M.s *** indicates difference between groups at the p<0.001
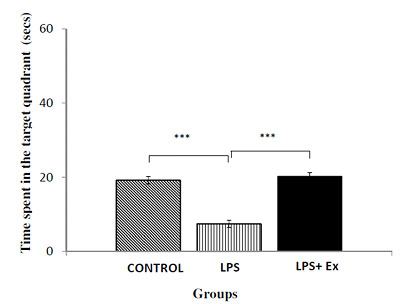
The adolescent physical (running) exercise attenuates cognitive deficits caused by prenatal LPS exposure.
Significant main effect of running exercise were present during the five days training period of MWM test, in which rats of LPS+Ex group showed significant decrease in latency to reach the escape platform, i.e. lesser duration to reach the platform, compared to the LPS group [Table/Fig-1]. However, except day 4 of training period, there were no significant difference noted between control and LPS+Ex group in the training days. In the memory retention test, with removal of platform, in comparison with the rats of LPS group, the LPS+Ex group rats remembered the location of platform and spent significant longer duration in the target quadrant in search of escape platform (Post-hoc, p<0.001). Conversely, between control and LPS+Ex group there was no significant difference observed in the memory retention test [Table/Fig-2].
Discussion
Gestational infections induced inflammation is associated usually with preterm labour and brain damage, such as white matter damage in the neonates, cerebral palsy, etc [22,23]. It is uncertain if the children, who were exposed to maternal inflammation during gestation, born without any clinical manifestation of cognitive paradigm remain normal in their lifespan [14]. In this study, we used LPS induced maternal inflammation model to explore its effect on spatial performance of young adult offspring.
Learning and memory are essential components of cognition. The first finding of this study demonstrate that young adult rats (2-month-old), exposed to LPS induced inflammations during their intrauterine life suffered from spatial learning and memory deficits. It was confirmed distinctly by MWM performance i.e. longer duration to reach escape platform during training sessions. The rats of control and LPS+Ex groups, whereas, exhibited shorter duration to reach escape platform. Despite trial sessions, the rats of LPS group were unable to hark back the location of escape platform during the memory retention test. This delineates decrement in the learning and spatial navigation performances of the litters treated prenatally with LPS. This finding is consistent with the study of Hao et al., who showed deficits in spatial performance of 3 and 10-month-old rats exposed to LPS induced inflammation during early and mid-gestation [14]. Graciarena et al., demonstrated that prenatal exposure to LPS inflammation during the mid and late gestation caused deficient in novel object recognition (NOR) performance in 2-month-old offspring, which are again consistent with the behavioural results of the current study [9].
The present study also provides the evidence that physical exercise during the adolescence period, a critical period when growth spurt occurs in brain, till the PND 45 where there is apparent dendritic arborization and synaptic connections formations in the stratum radiatum and moleculare layer of hippocampus [24,25], mitigates the cognitive impairment induced by LPS. In the retention test of MWM, the rats of LPS+Ex group spent longer duration in the quadrant where the escape platform used to be in training days, when compared with the LPS group. This indicates that early postnatal running exercise potentially attenuates cognitive impairment resulted due to prenatal LPS induced inflammatory insults in offspring albeit maternal inflammation impairing the spatial performance. Although the beneficial effect of physical exercise in altering the hippocampal volume [26], attenuating the cognitive impairment resulted due to stress [27] in adult and aged rodents are well documented, evidences elucidating the potential attenuating influence of early postnatal physical exercise, in young adult rats, on adverse outcome of prenatal insults such as prenatal inflammation, stress, etc. are dearth.
Previous studies also showed that physical exercises improved behavioural deficits in schizophrenia-like immune model by ploy I:C in 3-month-old mouse and restored impaired adult hippocampal neurogenesis [28]. Studies by Van Praag et al., showed that voluntary physical exercise increases long-time potentiation and neurogenesis in the hippocampus of different age group rodents thereby enhancing spatial learning and memory performances [29]. Significant improvements in cognitive function has been observed following moderate aerobic activity in aged humans [30]. The present study showed the beneficial role of early postnatal i.e. adolescent physical exercise in palliating the cognitive deficit, in young adult rats, resulting from exposure to LPS induced inflammatory insults during intrauterine growth period.
Conclusion
Collectively, our results demonstrate that inflammation during gestation impairs offspring’s spatial memory and learning abilities. Whereas, early postnatal physical exercise attenuates, to higher extent, cognitive impairment resulted from exposure to LPS induced inflammation during intrauterine growth period. However, further studies using histopathological and biochemical analyses will certainly facilitate to evaluate the correlation among structural alteration in offspring’s brain and their behavioural outcome in response to prenatal inflammatory insults and postnatal physical exercise.