Preloading with crystalloids has been a commonly employed technique to counteract spinal induced hypotension in parturients posted for caesarean section [1]. However, there is a concern regarding the safety and efficacy of this technique as there are studies which suggest no benefit of crystalloid preloading [2]. In addition, excessive fluid preloading may induce pulmonary oedema [3]. Fluid co-loading appears to be more physiological and rational approach as the maximal effect can be achieved at the time of onset of the block [4]. Recently, there have been few studies which indicate that co-loading may be equally effective as preloading with colloid [5,6]. However, there is no study where crystalloid preloading has been compared with colloid preloading and colloid co-loading. With this background, the present study was planned to study the effects of preloading using crystalloids and colloids with co-loading using colloid.
Materials and Methods
The study was conducted at a tertiary level hospital between January 2008 to December 2009 after approval by the Hospital Ethics Committee and written informed patient consent. Ninety ASA grade I/ II parturients with full term (36-40 weeks of gestation) uncomplicated singleton pregnancy scheduled for elective lower segment caesarean section under spinal anaesthesia were randomly allocated using a computer generated program to one of the three Groups of 30 each [7].
Any patient with fetal distress, ante-partum haemmorhage, pregnancy induced hypertension, diabetes mellitus, multiple gestation or significant cardiorespiratory disorder or intrapartum cardiomyopathy was excluded from the study.
Group-A (n=30): Parturients were given 10 ml/kg of colloid i.e. 6% Hydroxyethylstarch (HES) 20 minutes prior to administration of spinal anaesthesia (Colloid preloading).
Group-B (n=30): Parturients were given 10 ml/Kg of colloid i.e. 6% Hydroxyethylstarch (HES) by rapid infusion in 10 minutes immediately after administration of spinal anaesthesia (Colloid co-loading).
Group-C (n=30): Parturients were given 10 ml/kg of Ringer’s Lactate 20 minutes prior to administration of spinal anaesthesia (Crystalloid preloading). After a thorough pre-anaesthetic evaluation, all parturients were pre-medicated with oral tablet ranitidine 150 mg, the night before the caesarean section and 150 mg oral ranitidine 90 minutes prior to the elective caesarean section on the day of caesarean section.
Parturient was shifted to operating room in supine position with slight left lateral tilt. Oxygen at the rate of 5 l/min was given by a face mask. Intravenous access was secured with 18 gauge cannula in non dominant hand. With the parturient comfortably at rest in the supine position with slight left tilt, baseline heart rate, blood pressure, respiratory rate and oxygen saturation (SpO2) were taken.
Parturient was then turned to left lateral position and under all aseptic precautions, spinal anaesthesia was administered using a 26-gauge Quincke’s needle injecting 2.2 ml of 0.5% hyperbaric bupivacaine in the subarachnoid space at L3-L4 intervertebral space. Parturient was then immediately placed supine with slight left lateral tilt. Fluid administration was as per the Grouping based on the randomly generated numbers by a computer program. Ten minutes after induction of spinal anaesthesia, normal saline was administered in all the three Groups at the rate of 200 milliliters per hour which was adjusted according to needs of the patient and total fluid administered was recorded. The level of block was assessed by cold temperature discrimination using a spirit soaked cotton ball after 5 minutes. All parturients received oxygen by mask until delivery of baby. Haemodynamic parameters were recorded at every 5 min intervals till the end of caesarean section.
Hypotension was defined as systolic blood pressure (SBP) < 80% of baseline recording [8]. Hypotension was treated by increasing rate of fluid infusion and I/V bolus dose of ephedrine 5 mg until the pressure had returned to within 20% of baseline value. The number of episodes of hypotension were recorded along with number of doses and amount of ephedrine used. Haemodynamic parameters were recorded at every 5 min intervals till the end of caesarean section. At the end of procedure, parturient was shifted to recovery room. Monitoring of HR, NIBP, ECG, RR and SpO2 was continued in postoperative period every 15 minutes for 1 hour, ½ hourly for next 6 hours and then 1 hourly for next 5 hours. Patients were observed for signs of fluid overload like complain of respiratory distress or crepitations in chest, any allergic reactions to the fluids used and for excessive bleeding or oozing from the surgical field.
Statistical Analysis
After the completion of study, results obtained were tabulated and analysed using ANNOVA for Quantitative Analysis and for Qualitative Analysis, chi-Square test and z-test were used. p-value of less than 0.05 was considered significant. Also, in case of significant p-value, the critical difference (C.D.) was calculated and compared with the difference in mean values of different Groups. If the difference between mean values was higher than the critical difference, then it was considered statistically significant.
Results
There was no significant difference in the three groups with respect to mean age, height, weight and gestational age. Majority of the patients had no previous history of caesarean section in all the three Groups. Level of sensory block was similar in all the three Groups with no statistically significant difference [Table/Fig-1].
| BCC subtype | Group I | Group II | Group III | p-value |
|---|
| Mean age | 27.97±5.01 | 26.90±2.94 | 27.30±3.68 | 0.163 |
| Mean weight | 62.60±4.85 | 64.77±4.27 | 63.47±3.78 | 0.090 |
| Mean height | 162.35±5.58 | 162.24±4.60 | 162.8±5.30 | 0.357 |
| Gestational age | 38.08±1.24 | 38.31±0.99 | 38.35±1.08 | 0.183 |
| Previous cesarean (%) | 23.33 | 20.00 | 26.67 | 0.270 |
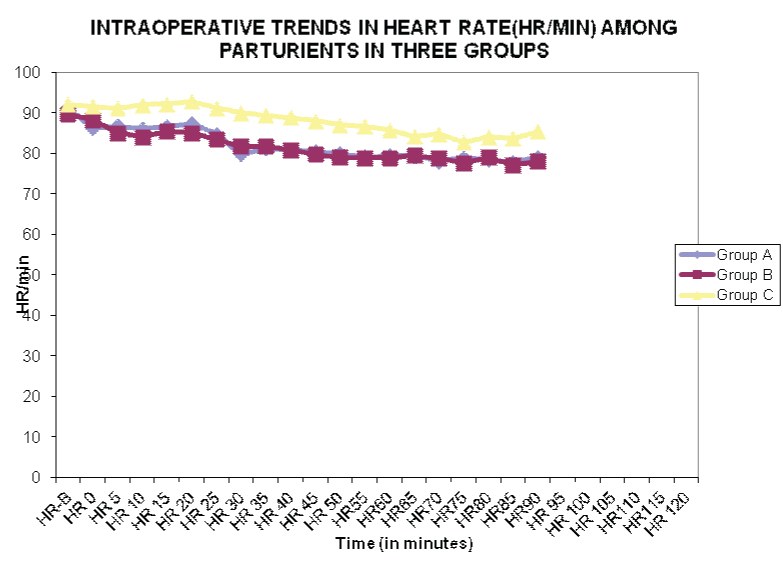
The mean values of baseline heart rate in Group A, Group B and Group C were statistically similar at 91.07±9.51, 89.67±10.49 and 92.10±10.40 per minute, respectively (p>0.05). In all the three Groups, the heart rate was found to have decreased from baseline at all time intervals intra-operatively. Mean Heart rate was higher in Group C as compared to Groups A and B and the difference was statistically significant at most time intervals except at 5, 10 and 20 minutes [Table/Fig-2].
Graphical representation for intraoperative trends in heart rate
The mean baseline value of mean arterial pressure (MAP) in Groups A, B and C was 89.33±6.11, 89.69±4.72 and 90.00±8.32 mmHg respectively which was statistically similar in all the three Groups (p>0.05). MAP decreased in all three Groups after induction of anaesthesia. However, between 5 to 55 minutes, at every 5 minute interval, MAP in Group C was lower as compared to Groups A and B and the difference was statistically significant. InterGroup comparison revealed that MAP between Group A and B were similar with no statistically significant difference. After 55 minutes, the difference in MAP was found to be insignificant between all the Groups at all time intervals (p-value > 0.05) [Table/Fig-3,4].
Intraoperative Trends in Mean Arterial Pressure (MAP)
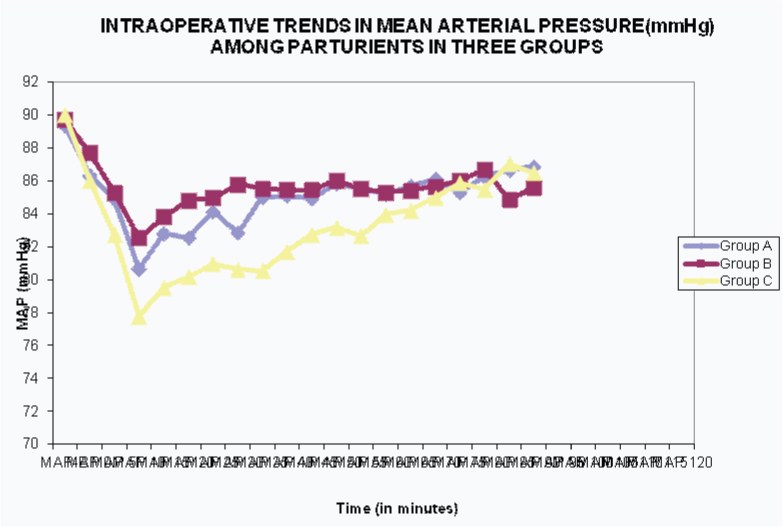
Table showing intra-operative trends in Mean Arterial Pressure (MAP)
| Time | Group A | Group B | Group C |
|---|
| MAP-B | 89.33 | 89.69 | 90 |
| MAP 0 | 86.33 | 87.69 | 86 |
| MAP 5 | 84.87 | 85.29 | 82.74 |
| MAP 10 | 80.66 | 82.55 | 77.77 |
| MAP 15 | 82.82 | 83.82 | 79.53 |
| MAP 20 | 82.53 | 84.8 | 80.18 |
| MAP 25 | 84.16 | 84.98 | 80.96 |
| MAP 30 | 82.84 | 85.78 | 80.62 |
| MAP 35 | 85.02 | 85.53 | 80.53 |
| MAP 40 | 85.09 | 85.44 | 81.71 |
| MAP 45 | 84.93 | 85.46 | 82.76 |
| MAP 50 | 85.82 | 86.02 | 83.16 |
| MAP 55 | 85.53 | 85.51 | 82.67 |
| MAP 60 | 85.22 | 85.31 | 83.96 |
| MAP 65 | 85.66 | 85.41 | 84.2 |
| MAP 70 | 86.12 | 85.67 | 84.99 |
| MAP 75 | 85.27 | 86 | 85.89 |
| MAP 80 | 86.27 | 86.71 | 85.48 |
| MAP 85 | 86.67 | 84.86 | 87.04 |
| MAP 90 | 86.87 | 85.56 | 86.48 |
Lowest MAP was seen at 10 minutes in all the three Groups, which was 80.66±10.58, 82.55±6.09 and 77.77±7.57 mmHg in Groups A, B and C respectively. Thus, lowest MAP was seen in Group C and the difference was statistically significant as compared to Groups A and B. Minimal MAP in Groups A and B were statistically similar [Table/Fig-3,4].
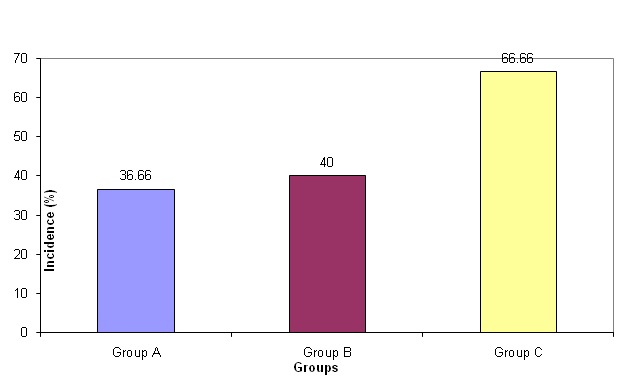
The incidence of hypotension was highest in Group C at 66.66% compared to 36.66% in Group A and 40% in Group B. InterGroup comparison revealed that the difference in incidence of hypotension was statistically significant between Groups A and C and B and C whereas, it was not significant between Groups A and B [Table/Fig-5].
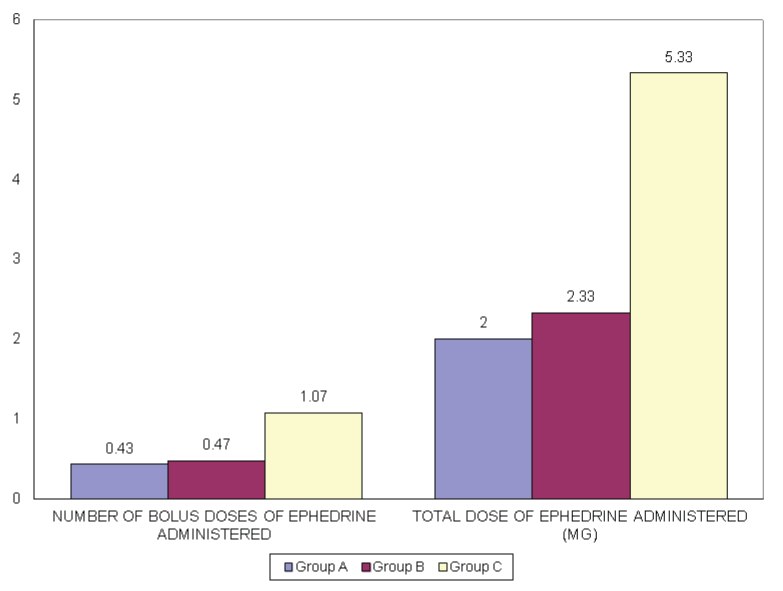
The mean number of bolus doses of ephedrine administered in Group A, B and C was 0.43±0.63, 0.47±0.63, 1.07±0.91 respectively and the mean total dose of ephedrine administered was 2.00±2.82, 2.33±3.14 and 5.33±4.54 in Groups A, B and C respectively. Thus maximum ephedrine dose was required in Group C and the difference was statistically significant [Table/Fig-6]. There was no statistically significant difference between ephedrine administration in Groups A and B. All the three Groups received similar amount of fluid intraoperatively and there was no significant difference in the amount of Intravenous fluid received among the three Groups.None of the patients was observed to have signs of fluid overload or pulmonary oedema or any adverse drug reactions to colloids. Similarly, none of the patients had excessive bleeding or oozing.
Number of boluses and total dose of ephedrine administered in the three Groups
Discussion
Preloading is a commonly employed technique to counteract hypotension following spinal anaesthesia. However, its effectiveness has been doubted as the incidence of hypotension still remains high [9]. For this reason some investigators have attempted co-loading [5,6]. In this study, we set out to compare colloid co-loading vs colloid and crystalloid preloading for prevention of spinal induced hypotension. Mean Heart rate decreased in all the three Groups, however, mean heart rate at most intervals was greater in Group C as compared to Groups A and B. This could be due to the fact that Group C received greater ephedrine boluses and ephedrine is known to cause tachycardia [10]. The MAP decreased in all the three Groups after administration of spinal anaesthesia and the lowest blood pressure was recorded at 10 minutes after spinal anaesthesia in all the three Groups. However, at most time intervals, MAP was lowest in Group C. The incidence of hypotension of 66.6 % was highest in Group C. The present study results are similar to those of Kundra et al., who reported 78 % hypotension in the crystalloid Preloading Group [11].
The ineffectiveness of crystalloid preloading has also been reported by Hofmeyr et al., who reported no benefit of preloading with 1L of RL even in parturients who were given low dose spinal anaesthesia [12]. Even higher dose of crystalloid preload are not effective, as reported by Tercanli et al., [13]. The possible reasons for the less efficacy of crystalloid solutions in prevention of spinal induced hypotension are that the crystalloid solutions have short intravascular half-life and rapidly leak into the extra cellular space [14]. Other reason is that preload is rapidly redistributed and may induce atrial natriuretic peptide secretion resulting in peripheral vasodilation followed by an increased rate of excretion of the preloaded fluid [15].
The incidence of hypotension in Groups A and B was significantly less than Group C. Thus colloid administration was more effective to prevent hypotension. The superiority of colloids to reduce hypotension has also been reported by Riaz et al., who showed that preloading with colloids is more effective than crystalloids [16]. Similarly, Dahlgren et al., noted a higher frequency of hypotension in crystalloid preload Group compared to colloid preload Group and observed that colloid preloading is superior to crystalloid in reducing the incidence of spinal induced hypotension [9].
Inter Group comparison revealed that there was no significant difference in the incidence of hypotension between Groups A and B. Our results correspond to those of Carvalho el al., who reported that the incidence of hypotension was similar in Groups receiving colloid Preloading and coloading Groups [5]. Similarly, Mercier et al., showed that loading fluid at the time of administering the intrathecal local anaesthetic (co-loading) might be a physiologically more appropriate and rational approach as the maximal effect can be achieved during the time of the block [17].
Our results are similar to those of Teoh et al., and Sayyid et al., who observed that the incidence of hypotension and vasopressor requirement was similar in the Groups receiving colloid preload and co-load [14,18]. Similarly, Banerjee et al., in a meta-analysis noted that the incidence of hypotension in the co-load Group was 59.3% compared with 62.4% in the preload Group [6].
In the present study, ephedrine consumption was found to be highest in Group C. However, both mean ephedrine consumption and total number of ephedrine boluses were similar between Groups A and B. These findings correspond to those of Dahlgren et al., who reported a greater need for ephedrine, in the colloid Preloading Group as compared to crystalloid Group [9]. Similarly, Carvalho et al., showed that there was no difference in the intraoperative ephedrine requirement in the colloid preloading and co-loading Group [5]. In postoperative period, during study period of 12 hours no episode of hypotension was reported, so ephedrine was not administered in any of the Groups. Thus, our study indicates that both Preloading and co-loading with colloids are an effective method for prevention of hypotension as compared to preloading with crystalloids. However, one limitation of this study is that the effects on neonate were not observed, secondly, adverse effects of colloids on coagulation were not studied using laboratory tests. Only clinical examination was done looking for oozing or excessive bleeding. The reason for not using laboratory investigations was that we were using colloids very much within the recommended dosages and colloids interfere with coagulation when used in excessive doses.
Conclusion
The present study concludes that both colloid preloading and co-loading are equally effective for prevention of maternal hypotension following spinal anaesthesia. So there is no need to postpone the surgery in order to wait for preloading to take place. Secondly, crystalloid preloading is inferior when compared to colloid preloading or co-loading for this purpose.