Morphometrical Analysis of Developing Cochlear Ganglion Neurons: A Light Microscopic Fetal Study
Madhu Sethi1, Sabita Mishra2, Neelam Vasudeva3, J.M. Kaul4
1 Assistant Professor, Department of Anatomy, Dr. Baba Sahab Ambedkar Medical College & Hospital, Rohini, New Delhi, India.
2 Professor, Department of Anatomy, Maulana Azad Medical CollegeNew Delhi, India.
3 Director Professor and Head, Department of Anatomy, Maulana Azad Medical College, New Delhi, India.
4 Professor and Head, Department of Anatomy, Dr. Baba Sahab Ambedkar Medical College & Hospital, Rohini, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Madhu Sethi, Assistant Professor, Department of Anatomy, Dr. Baba Sahab Ambedkar Medical College & Hospital, Rohini-110085, New Delhi, India.
E-mail: madhusethi567@gmail.com
Background and Aim
The cochlear or spiral ganglion neurons are the initial bridge between the external world of sound and its discernment in the brain. As the developing human fetal cochlea is known to start functioning in mid gestational period, its anatomical details when compared with adults could vary with each gestational age. The aim of current study was to assess morphometrical parameter of developing human fetal cochlear ganglion neurons and comparison of data in each gestational period.
Materials and Methods
Ten aborted human fetuses from 14th to 28th weeks of gestation were procured from Department of Obstetrics and Gynaecology of associated hospital, after obtaining ethical clearance and were processed for studying under light microscope. Area of neurons from each gestational age was measured on histophotomicrographs using image Proplus software. Standard statistical method was used to calculate area range and percentage of small and large ganglion neurons.
Results
The neuronal area increased progressively in successively higher gestation age fetuses. In the fetus belonging to lowest gestational age the area ranged from 4-37μm2 while in highest gestational age fetus its range was 10-58.3μm2. The small ganglion neurons were higher in 14 weeks (65.5%) fetuses and 16-20 weeks (81.03%) fetuses, while in higher gestational age fetuses’ large ganglion neuronal population was higher (62-66%).
Conclusion
A baseline morphometrical representation of fetal cochlear ganglion neurons could be of relevance in advanced human experimental studies on effect of neurotrophic factors in human fetuses with congenital deafness. It has been found that these factors directly influence neuronal maturation assessed by progressive increase in soma size and survival.
Development, Fetus, Neurons, Spiral ganglion, Quantitative
Introduction
The cochlear ganglion or spiral ganglion neurons of the cochlea represent a distinct and sequestered population of primary sensory neurons of precarious relevance for transmission of auditory stimuli to the brain. The adult human cochlear ganglion consists of two well characterized bipolar neuronal population groups known as: Type I and Type II [1]. These groups are recognized as large and small ganglion neurons respectively [1]. The classification has been based on soma size, relative abundance, cytologic traits, and characteristics of central and peripheral processes [2–4]. The large Type I neurons constitute 90-95% of the total neuronal population and their perikarya have a diameter of 22-34μm with a length of 22-64μm. In comparison to them the smaller Type I ganglion neurons are 5-10% of the total percentage of cochlear ganglion neurons. Their diameter ranges from 8-14μm and length varies from 15-21μm [1,5,6].
Though morphometrical details of adult human cochlear ganglion neurons are well known, there is dearth of data on such details in developing human fetuses. Till now various studies have been conducted on developing animals to understand morphometrical maturation of cochlear ganglion with detailed study on experimentally deafened animals to provide an insight for cochlear implant studies [7–10].
Morphological characteristics of maturing human fetal cochlear ganglion have been studied earlier describing anatomical specificities [11–13], but the data on morphometrical analysis of developing human cochlear ganglion neurons have been rarely documented. Thus, the current study was focused on morphometrical analysis of developing human fetal cochlear ganglion neurons and recording of data from each gestational period.
Materials and Methods
Fetus Collection and Preservation/Fixation
This was an observational study conducted on ten aborted human fetuses aged between 14-28 wk of gestation obtained during 2008-2010, from the Department of Obstetrics & Gynaecology, with prior approval from the Institutional Ethical Committee.
Fetuses less than 20 wk gestation (WG) were collected from cases where Medical termination of pregnancy was conducted under the MTP Act. Fetuses with gestational age more than 24 wk were obtained from spontaneous abortions and still birth. Fetuses with any congenital anomaly, putrefied or macerated fetuses and those belonging to mothers with any medical illness during pregnancy were excluded from the study. To minimize postmortem changes the fetuses were immediately fixed in 10% buffered formalin and thus preserved by immersion fixation method. For determining fetal age, relevant parameters like weight, Biparietal diameter, Crown rump length, Crown heel Length and Foot length were measured [14].
Tissue Preparation and Staining
After fixation brain was removed and petrous part of temporal bone was dissected out and kept in 10% buffered formalin for one week. Specimens from higher gestational ages were decalcified in 10% EDTA. Specimens were labeled and processed for paraffin embedding. Seven micron thick serial sections were generated on a rotary microtome with the anterior surface of the petrous temporal bone as the cutting surface along its superior border. The sections were stained with 1% cresyl violet stain and were studied subsequently.
Morphometrical Analysis
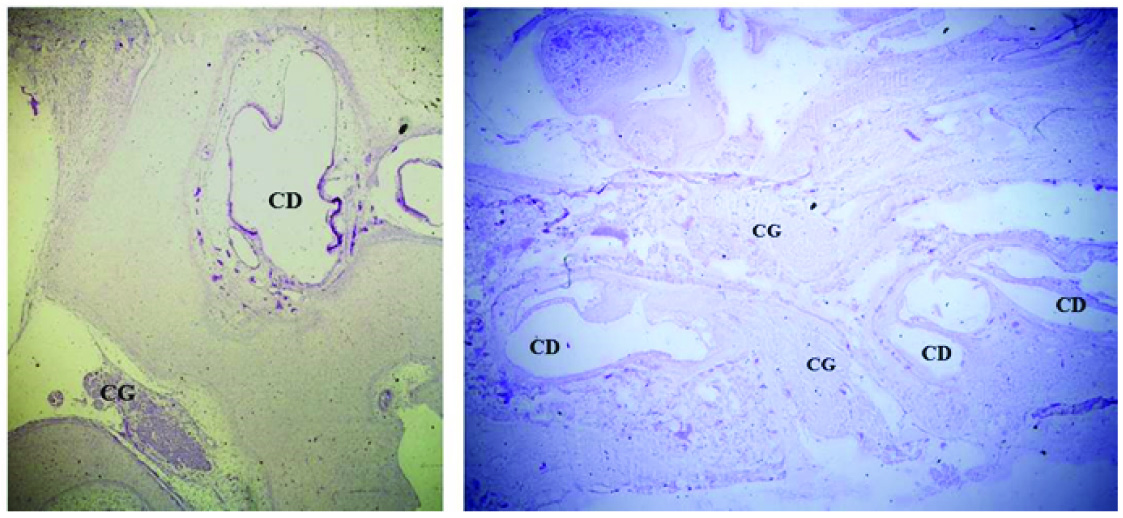
Observations were made using a BX61computerised microscope under 100X objective lens and images were captured by DP71 camera and further analysed by image Proplus MC6 software. The ganglion was identified by its proximity to the developing cochlear duct as a cluster of neurons [Table/Fig-1]. Morphometrical parameter was observed under oil-immersion. Every tenth serial section was included for observation. Area of hundred neurons in sections of each gestational age specimen was measured as micron meter square. The data was tabulated in excel sheets and assessed.
Histophotomicrograph of cresyl violet stained sections of fetal temporal bone, showing cochlear ganglion (CG) lying in relation to the developing cochlear duct (CD) [1a:20 weeks of gestation, 1b: 24weeks of gestation; magnification 2Xobjective
Bar graphs were made for all the data recorded and in preliminary analysis each histogram was scanned visually. The bar diagram plotted for each gestational age fetus provided a visual display of the data for segregating the quantitative variable obtained into two comparable groups of small and large ganglion neurons [15] . The reference unit was different for each gestational age fetus since the neuronal population was in the process of maturation. Hence for each gestational age fetuses small and large ganglion neuronal population were segregated independently in accordance to the bar graphs plotted. Area range of neuronal population group and percentage of small and large ganglion neurons were calculated using standard basic statistical method with SPSS version 15.
Results
The aborted fetuses belonged to 14 wk, 16 wk, 20 wk, 24 wk, 26 wk and 28 wk of gestation. Only one fetus from 14 and 16 wk of gestation was collected, however two fetuses from each subsequent age of gestation were procured.
At oil immersion magnification the ganglion neuronal group was seen as a cluster of neurons, supporting cells and intervening nerve processes. The nucleus and nucleolus were clearly visible in each histophotomicrograph [Table/Fig-2].
Histophotomicrograph of the cochlear ganglion neurons at 28 weeks gestation under oil immersion lens [100X objective]

It was observed that the neuronal area ranged from 4-37μm2 in the fetus belonging to lowest gestational age and the area range in fetus from highest gestational age was 10-58.3μm2 [Table/Fig-3]. The observation tabulated [Table/Fig-3] along with the bar graphs as histogram depicting the results are shown in [Table/Fig-4]. The results showed that the percentage of small ganglion neurons was higher in 14 wk and 16-20 wk fetuses, while in higher gestational age fetuses large ganglion neuronal population was higher [Table/Fig-5].
Table showing area range of small and large ganglion neurons at each gestational age along with their percentage with the neurons showing a nucleus and nucleolus
| Week of gestation (weeks) | Area range (μm2) | Small ganglion neurons | Large ganglion neurons |
|---|
| Percentage of neurons | Area range (μm2) | Percentage of neurons | Area range (μm2) |
|---|
| 14 | 4-37 | 65.5% | 4-20 | 34.5% | 21-40 |
| 16-20 | 2-27 | 81.03% | 2-15 | 18.9% | 15.1-27 |
| 24 | 4-47.7 | 38% | 4-23.4 | 62% | 25.2-47.7 |
| 26 | 6-50.2 | 36.5% | 6-27.5 | 63.5% | 30.1-50.4 |
| 28 | 10-58.3 | 34% | 10-30 | 66% | 32.2-58.3 |
Histogram depicting distribution of the value of area of the neurons along X-axis and the number of neurons along Y-axis for respective gestational age fetus. [4a:14weeks; 4b:16-20weeks; 4c: 24weeks; 4d: 28weeks]

Cone graphs showing percentage of small and large ganglion neurons at 14,16,24 and 28 weeks respectively

Discussion
This was a light microscopic study on serial sections of temporal bone to provide numerical data on the area of developing cochlear ganglion neurons. There are very few studies on human fetal cochlear ganglion neuronal morphometry.
In an earlier study on human fetal cochlear ganglion neurons belonging to various gestational ages, the morphometrical findings given as area range at each turn of cochlea, stated that neuronal population had an area range of 12 to 48 μm2 in all turns at 32 wk (270mm CRL) gestation. It included all ganglion neurons as Type II. In the basal and middle turns of neonate, two neuronal populations were found: a) Small neurons (mean area 20μm2 ) and b) large neurons (mean area 100μm2) [11]. It was specified that small ganglion neurons might be the precursors of large ganglion neurons and as maturation occurs the small ganglion neurons could have differentiated into large ganglion neurons. The fetus of highest gestation age in our study was of 28 wk gestation, in which the population of large ganglion neurons (66%) was more than the small neurons. In this gestational age fetus area of large ganglion neurons ranged from 32.2 to 58.3μm2 and those of small ganglion neurons ranged from 10 to 30 μm2. At 16-20 weeks of gestation more of small neurons were seen in comparison to large neurons suggesting that more neurons could have migrated into the ganglion area and may later grow to form the larger neurons as suggested by earlier study [11]. The difference obtained in neuronal area values could be attributed to the varying factors influencing the development about which no definite causation can be accredited with confirmation. With progressive development increase in soma size also strengthens the fact that concomitant morphological maturation of neurons is occurring with its functional maturation as observed in other corresponding literatures [12,13].
In present study, based on the shape of bar graphs which showed a bimodal distribution, the neuronal collection was segregated as small and large ganglion population in each gestational age sample [15]. In an earlier ultrastructural morphometrical study on adult human cochlear ganglion neurons a possibility of five subgroups amongst the group of large and small cells were observed. In middle and upper middle turns three groups of cells has been assumed based on ultrastructural morphometrical analysis [16].
Comparable electron microscopic study on morphometry and distribution of cell types in human neonate has shown that segmental density of spiral ganglion neurons is higher in neonates than in adults, with higher prevalence of Type II ganglion neurons than reported in adults [17]. A clear differentitation of Type I and Type II ganglion neurons can be seen in human neonate and with age the prevalence of Type II ganglion neurons is known to decrease particularly in middle and apical turns [17]. In our study, two different neuronal population groups were observed on light microscopic morphometrical basis but no segregation was done into Type I and Type II neurons specifically. To differentiate Type I and Type II cochlear ganglion neurons special immune markers and ultrastructural details are required [8,9].
The development and maintenance of cochlear ganglion neurons and its peripheral process requires action of various growth factors such as neurotrophins, fibroblast growth factors and glial cell line-derived growth factors [18–22]. Recent treatment strategies of bionic cochlear implant use these growth factors to aid survival of cochlear ganglion neurons in deafened ear and enhancing therapeutic outcomes [10]. Many experimental studies have shown that whenever there is loss of neurotrophic support from these factors, the ganglion neurons appear unhealthy with smaller soma [19] and pyknotic, misshapen nuclei [23–25]. Other experimental studies on deafened animals have shown that on treatment with exogenous neurotrohic factors, the surviving spiral ganglion neurons appeared larger, and healthier with well defined nucleus, nucleolus and abundant Nissl substance [19,26]. The survival effect of these cell growth promoting factors is assessed by evaluation of neuronal soma size and neuronal density and such studies are still at experimental level in lower animals. This study can provide a baseline morphometrical data of normal developing human fetal cochlear ganglion neurons to be comparable with congenitally abnormal cochlear ganglion neurons occurring due to any prenatal ototoxic insult affecting expression of growth factors which are normally expressed by the hair cells and cochlear ganglion neurons in prenatal period [27]. To fully apprehend the application of advancing treatment strategies using such growth factors a more detailed morphometrical data characterizing other maturation changes of developing human cochlear ganglion neurons is required.
Limitation
Advanced microscopes like confocal microscope, electron microscope are helpful in getting finer details of neurons. However, these were not available in the study institute.
Due to precarious availability of human fetuses, the sample size could not be increased much. The study was a part of thesis and hence was time bound. Also, one fetus is procured in two months from the associated hospital. Rapid autolysis on neuronal tissue also requires discarding most of the samples which were collected.
Conclusion
Normal maturation of developing human cochlear ganglion neurons comprises of progressive increase in size of neuronal soma. The neuronal population is broadly divisible into large and small ganglion neurons with the small ganglion neurons exceeding during initial stages of development and the large ganglion neurons seen more in higher gestational age fetuses. Upcoming treatment strategies for congenital deafness using growth factors require a detailed baseline morphometrical data to describe comparable changes of developing human cochlear ganglion neurons.
[1]. Ota CY, Kimura RS, Ultrastructural study of human spiral ganglionActa Otolaryngol 1980 89:53-62. [Google Scholar]
[2]. Spoendlin H, Degeneration behaviour of the cochlear nerveArch Klin Exp Ohren Nasen Kehl kopfheilkd 1971 200:275-91. [Google Scholar]
[3]. Spoendlin H, The innervation of the cochlea receptor. In: Moller, AR., editorMechanisms in Hearing 1973 New YorkAcademic Press:185-229. [Google Scholar]
[4]. Kiang NY, Liberman MC, Gage JS, Northrup CC, Dodds LW, Oliver ME, Afferent innervations of the mammalian cochlea. In: Bolis L, Keynes RD, Maddrell HP, editorsComparative Physiology of Sensory Systems 1984 CambridgeCambridge University Press:143-61. [Google Scholar]
[5]. Rosenbluth J, The fine structure of acoustic ganglia in the ratJ Cell Biol 1962 12:329-59. [Google Scholar]
[6]. Spoendlin H, Differentiation of cochlear afferent neuronsActaOtolaryngol 1981 91:451-56. [Google Scholar]
[7]. Schwartz AM, Parakkal M, Gulley RL, Postnatal development of spiral ganglion cells in the ratAm J Anat 1983 167(1):33-41. [Google Scholar]
[8]. Hafidi A, Romand R, First appearance of Type II neurons during ontogenesis in spiral ganglion of the rat an immunocytochemical studyDevelopmental brain research 1989 48:143-49. [Google Scholar]
[9]. Berglund AM, Ryugo DK, Neurofilament antibodies and spiral ganglion neurons of the mammalian cochleaJ Comp Neurology 1991 306(3):393-408. [Google Scholar]
[10]. Budenz CL, Pfingst BE, Raphael Y, The Use of Neurotrophin Therapy in the Inner Ear to Augment Cochlear Implantation OutcomesAnat Rec (Hoboken) 2012 295(11):1896-908. [Google Scholar]
[11]. Sánchez Del Rey A, Sánchez Fernández JM, Martínez Ibarguen A, Santaolalla Montoya F, Morphologic and morphometric study of human spiral ganglion developmentActaOtolaryngol 1995 115(2):211-17. [Google Scholar]
[12]. Sethi M, Mishra S, Kaul JM, Vasudeva N, Morphogenesis and maturation of synapses in developing human cochlear ganglionJ Morphol Sci 2013 30(4):289-98. [Google Scholar]
[13]. Bibas A, Hornigold R, Liang J, Development of spiral ganglion in human fetusFolia morphol 2006 65(2):140-44. [Google Scholar]
[14]. Sailaja K, Ahuja RK, Gopinath G, Biparietal diameter: a useful measure for determining gestational age of human abortusesNatl Med J India 1996 9:165-67. [Google Scholar]
[15]. Jackson S, Research Methods and Statistics: A Critical Thinking Approach 2011 4th EditionWadsworthCengage Learning [Google Scholar]
[16]. Nadol JB Jr, Burgess BJ, Reisser C, Morphometric analysis of normal human spiral ganglion cellsThe Annals of Otology, Rhinology and Laryngology 1990 99(5 Pt 1):340-48. [Google Scholar]
[17]. Chiong CM, Burgess BJ, Nadol JB Jr, Postnatal maturation of human spiral ganglion cells: light and electron microscopic observationsHear Res 1993 67(1-2):211-19. [Google Scholar]
[18]. Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM, Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factorsAnn N Y Acad Sci 1999 884:305-11. [Google Scholar]
[19]. Kanzaki S, Stöver T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervationJ Comp Neurol 2002 454:350-60. [Google Scholar]
[20]. Glueckert R, Bitsche M, Miller JM, Zhu Y, Prieskorn DM, Altschuler RA, Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factorJ Comp Neurol 2008 507:1602-21. [Google Scholar]
[21]. Defourny J, Lallemend F, Malgrange B, Structure and development of cochlear afferent innervation in mammalsAm J Physiol Cell Physiol 2011 301(4):C750-61. [Google Scholar]
[22]. Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B, The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of CortiHear Res 2011 278(1-2):21-33. [Google Scholar]
[23]. Ernfors P, Duan ML, ElShamy WM, Canlon B, Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3Nat Med 1996 2:463-67. [Google Scholar]
[24]. Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR, NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cellsNeuroreport 1996 7:889-94. [Google Scholar]
[25]. Gillespie LN, Clark GM, Bartlett PF, Marzella PL, BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effectsJ Neurosci Res 2003 71:785-90. [Google Scholar]
[26]. Staecker H, Galinovic-Schwartz V, Liu W, Lefebvre P, Kopke R, Malgrange B, The role of the neurotrophins in maturation and maintenance of postnatal auditory innervationAm J Otol 1996 17:486-92. [Google Scholar]
[27]. Vandenbosch R, Chocholova E, Robe PA, Wang Y, Lambert C, Moonen G, A role for the canonical nuclear factor-κB pathway in coupling neurotrophin-induced differential survival of developing spiral ganglion neuronsFront Cell Neurosci 2013 7:242 [Google Scholar]