Introduction
Endoscopic Retrograde Cholangio Pancreatography (ERCP) is an invasive procedure performed in remote locations under a continuum of anaesthetic depth, ranging from conscious sedation to deep sedation leading to general anaesthesia [1]. The major challenges involved are preservation of spontaneous respiratory efforts, shared airway, positional variations like lateral/semi prone or prone [2].
BiSpectral index (BIS) is a value derived from multiple EEG descriptors and provides a non invasive measure of level of sedation [1]. Targeting BIS within a specific range ensures additional safety during the procedure. Scores between 65-85 have been recommended for sedation [3].
Propofol is a widely used drug for ERCP sedation due to its pharmacological properties and rapid recovery profile [4,5]. In spite of its favourable profile, due to lack of analgesic properties, large doses may be needed for maintenance of anaesthetic depth especially in prolonged ERCP procedures which can cause cardio respiratory adverse effects [4].
Propofol requirement can be reduced with addition of adjuvants. Ketamine (NMDA antagonist) and Dexmedetomidine (selective α2 agonist) are sedatives having analgesic properties with opioid and anaesthetic sparing effects, without clinically significant respiratory depression [4,6-8].
Materials and Methods
After obtaining institutional ethics committee approval, on the basis of previous published studies [1], a sample size of 24 patients was required in each group in order to obtain 80% power (total of 72 Kunnavil6patients). Therefore, 75 patients were enrolled to compensate for possible dropouts. Patients in the age group 18-75y, of either sex, belonging to American Society of Anesthesiologists Physical Status I–III, were enrolled in the study after obtaining detailed written informed consent. The following patients were excluded from the study:-
• Critically ill patients admitted in ICU.
• Preexisting delirium/cognitive dysfunction.
• Hyponatremia (Serum Sodium <120 meq/l) (Risk of altered sensorium interfering with Mini Mental State Examination score).
• Evidence of hepatic encephalopathy, ascites.
• Hemodynamically unstable patients on inotropic support.
• Allergy to propofol/ egg.
• Hypo/Hyperkalemia (Potassium <3meq/lit or >5.5meq/l) (risk of dysrhythmias).
Group Allocation/Randomisation
Patients willing to participate in the study were allocated to 3 groups using computer generated random number table utilizing sealed envelope technique as:
Group DEXMEDETOMIDINE: receiving dexmedetomidine bolus (1μg/kg) followed by dexmedetomidine infusion (0.5μg/kg/h) along with variable propofol boluses.
Group KETAMINE: receiving ketamine bolus (0.25mg/kg) followed by ketamine infusion (5mcg/kg/min) along with variable propofol boluses.
Group CONTROL: receiving saline bolus followed by saline infusion along with variable propofol boluses.
Study Design (REG NO: ECR /215/Inst/Ker/2013)
The study was designed to be a randomized controlled prospective double-blinded study. After randomly being allocated to a respective group, an anaesthesia resident, who was uninvolved with the study but involved in the procedure, loaded up the drug according to the group the patient belonged to. A qualified anesthetist who was a part of the study and who was blinded to the group allocation then anesthetized the patient for the procedure and an independent observer blinded to the study performed the post procedure recovery scoring.
All patients posted for outpatient interventional ERCP in the ERCP suite (with backup OT and ICU care in the event of complications) underwent a thorough Pre – Anaesthetic evaluation including a Mini Mental State Examination and score was documented. Patients were advised to arrive nil orally for at least 6 h prior to procedure, pre medicated with oral Pantoprazole 40mg and Ondensetron 8mg on arrival to the endoscopy suite. An intravenous line (i.v.) was secured and started on maintenance intravenous fluid 0.9% sodium chloride/ringer lactate.
Standard anaesthesia monitoring in the form of 3 electrode ECG, Non Invasive Blood Pressure (NIBP), Pulse oximeter probe were connected and BiSpectral Index monitor (ASPECT A-2000 TM) with single use, disposable, low impedance BIS sensor consisting of three electrodes applied over the forehead using fronto temporal montage. The baseline variables were documented and continued to be monitored and documented every 5 min for the first 30 min and every 10 min there on till the end of procedure. Supplemental Oxygen was administered with nasal prongs at 3 litre/min. The total duration of the procedure, defined as the time taken from insertion of the endoscope to its removal, was also documented.
Intravenous premedication with Midazolam 0.05mg/kg, Hyoscine 0.3mg/kg and Pharyngeal topicalisation with 10 ml of 2% lignocaine gargle was given to patients in all 3 groups. No intravenous opioid was utilized in this study.
The study drug was loaded in 10 and 50 ml syringes (for bolus and infusion respectively) & labeled as “study drug”. The identity of the constituted drug in the syringe was not revealed to the patient, the anesthetist in charge of the case & the independent observer who recorded the post procedure variables. Depending upon the body weight, each patient received a bolus of the study drug, diluted up to 10 ml with saline followed by infusion of the same in a 50 ml using a syringe pump (Fresenius Kabi TM) the rate of which was controlled by the anaesthesia resident.
Bolus dose of the study drug was administered immediately after patient positioning followed by induction with propofol in a dose of 0.5-1.5 mg/kg, targeting BIS between 60-70. Once the target BIS was attained, the infusion of the scheduled study drug (as per group allocation) was started. An experienced Endoscopist (Gastroenterologist with minimum 5 y of endoscopy experience) then commenced the procedure. Any increase in the BIS value above the target range was managed by administering incremental i.v. propofol boluses (20 mg increments) and propofol bolus administration was with held when BIS <55.
At the completion of the procedure, further propofol boluses and background infusion of the scheduled drug was stopped and BIS value allowed equilibrating above 80. Patients oropharynx thoroughly suctioned, turned supine with head up tilt (15 degrees), allowed for complete recovery with end points being eye opening on command, ability to handle secretions, follow simple commands, hemodynamic stability, maintaining room air saturation >95% and attainment of BIS value >90.
Patients were shifted to the recovery room, kept in propped up position, oxygen supplementation via face mask (5lit/min), maintenance intravenous fluid (ringer lactate/ 0.9% saline) and continuation of standard monitoring was done.
Recovery characteristics were noted using Modified Aldrette Score (MAS) [9] & Observer Assessment of Alertness and Sedation score (OAAS score) [10] and patient was discharged after documenting time taken for attainment of MAS > 9 and an OAAS score > 4, along with a responsible attendant.
Outcome Measurements
Primary-The total propofol consumption in each study group
Secondary-1) Recovery profile of patients in each study group
2) Haemodynamic profile of patients in each study group
Statistical Analysis
All quantitative and continuous variables such as age, weight, vital parameters, utilizing descriptive statistics, were expressed as mean ± standard deviation. Mean differences between the groups were compared using ANOVA / Kruskal Wallis test. Mixed Model was used to distinguish the mean difference in hemodynamic variables among the 3 groups by taking treatment as fixed effect and time as random effect. p <0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 20(SPSS Inc. Chicago, Illinois, USA) and Microsoft Excel 2011 (Microsoft Corporation, Redmond, Washington, USA).
Results
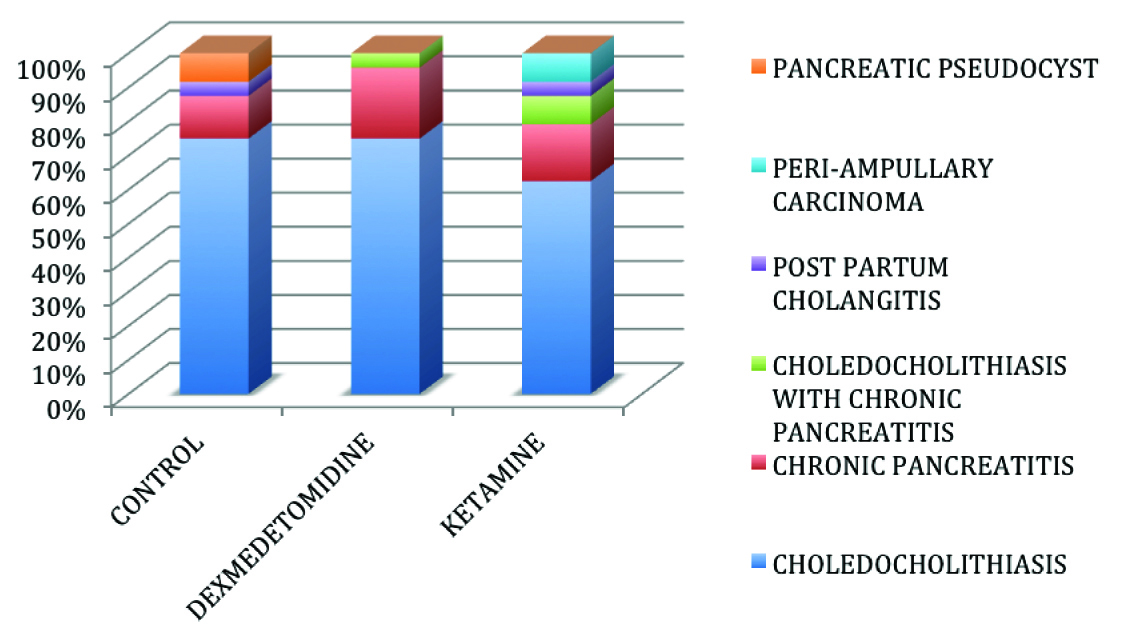
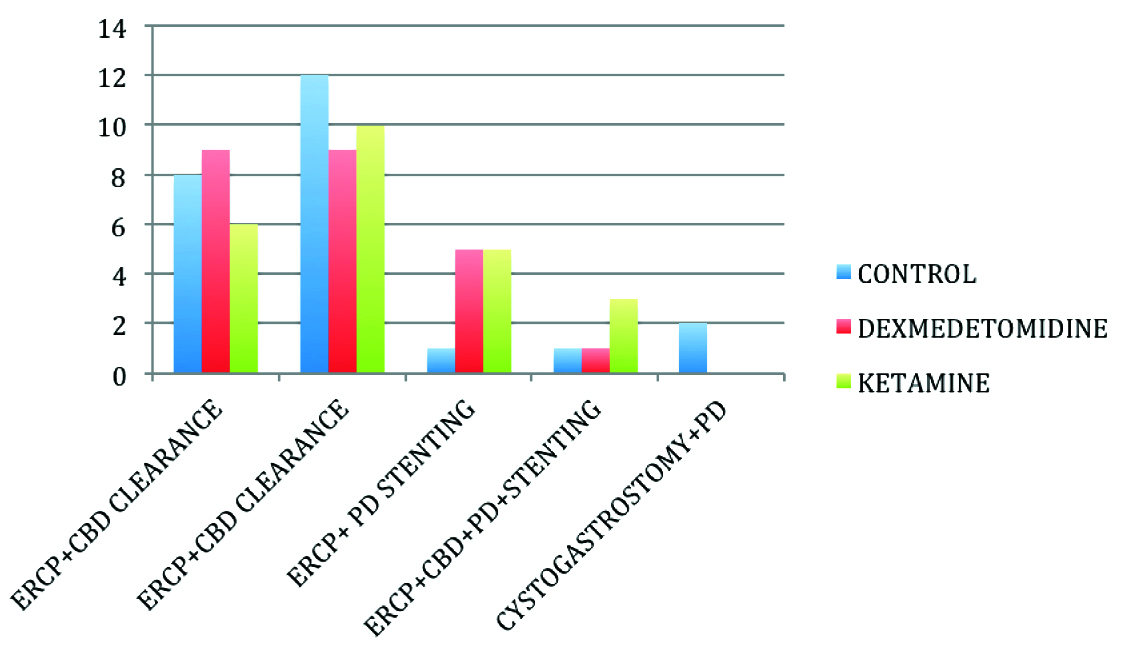
A total of 75 patients were enrolled in the study of which, 72 patients completed the study [Table/Fig-1]. Patients were comparable in all 3 groups with regard to age, weight, sex, ASA status, pre procedural Mini Mental Scores, Serum Sodium and duration of procedure [Table/Fig-2,3,4] and also with respect to the pre procedural diagnosis [Table/Fig-5]. The procedures performed in the 3 groups are depicted in [Table/Fig-6].
Primaryry Outcome
Propofol Consumption in each Study Group
The total propofol consumption was significantly higher in the control group (255.83 ± 114.2 mg) when compared to the Dexmedetomidine (162.5 ± 71.7 mg) and Ketamine groups (158.33 ± 66.89); p= 0.001 {Kruskal-Walli’s test & Bonferroni multiple comparison test} [Table/Fig-3,4]. No significant difference was found when dexmedetomidine and ketamine groups were compared against each other; p=1.000; {Bonferroni multiple comparison test} [Table/Fig-4,7].
Secondary Outcome Measures
Recovery Profile Assessment of Patients in 3 Groups
Patients in the control group attained a MAS > 9 and OAAS >4 much faster (7.5 ± 3.29 min for MAS and 6.88±2.49 min for OAAS) than patients in the dexmedetomidine group (16.6 ± 3.18 min for MAS and 16.67 ± 2.82 min for OAAS) and in ketamine group (10 ± 4.17 min for MAS and 8.75 ± 3.68min for OAAS). p<0.001(Kruskal Walli’s test) [Table/Fig-4,7].
The time taken to achieve MAS >9 and OAAS score >4 was significantly longer in the dexmedetomidine group when compared with the control and ketamine groups; p <0.001 {Bonferroni multiple comparison tests}. However, no statistical significance was found when the control and ketamine groups were compared against each other (p=0.54 for MAS and p= 0.108 for OAAS score) [Table/Fig-1,7].
Haemodynamic Profile of Patients in 3 Groups
The baseline Systolic & Diastolic Blood Pressures, oxygen saturation and BIS values were comparable among patients in the 3 groups [Table/Fig-8]. The average heart rates and Mean Arterial Pressures throughout the procedure were calculated and its distribution is as shown in [Table/Fig-9,10].
Mixed model analysis comparing the mean heart rates and Mean Arterial Pressures of patients in the 3 groups revealed a significantly lower heart rate in patients of Dexmedetomidine group when 9compared to the control and ketamine groups (p<0.001). Heart rates of patients in control and ketamine group were comparable to each other [Table/Fig-11,12] No statistical difference was found when the average Mean Arterial Pressures throughout the procedure were computed among the 3 groups and therefore an intergroup comparison was not performed [Table/Fig-11].
Discussion
ERCP has evolved from being a simple diagnostic procedure to becoming a therapeutic procedure with increased duration and com-plexity, requiring a high degree of patient co- operation. Sedation and analgesia to reduce pain, discomfort and stress in patients undergoing ERCP contribute to better patient tolerance and compliance. Studies have indicated that complications such as duodenal perforation and pancreatitis result as a consequence of poor patient cooperation manifested by restlessness and anxiety during the procedure [11,12]. With the advent of day care anaesthetic services, the trend has shifted to ERCP’s becoming an outpatient procedure.
With ERCP being routinely performed under conscious sedation, deep sedation or general anesthesia outside the comfort of the OT, the need for anesthetic depth monitoring becomes an essential armamentarium to ensure patient safety [5]. The Practice Committee of American Society for Gastrointestinal Endoscopy has stated that the use of EEG monitoring may have a role in the future for the delivery of sedation during selected endoscopic procedures [13]. Wehrmann et al., [14], found that mean propofol dose was significantly lower in the group of patients sedated with EEG guided monitoring as compared with that of control group. The first and only technology approved by the U.S. Food and Drug Administration (1996), for marketing as an EEG based monitor of anaesthetic effect is the BiSpectral (BIS) analysis derivative [3]. BIS monitoring during ERCP was chosen in our study for optimal sedation guidance and to prevent sedation related complications. Meta-analysis of ambulatory surgery studies showed that use of BIS monitoring significantly reduces the anaesthetic consumption by 19% [15]. No intra procedural sedation scores were used in this study due to an objective measure available in the form of BIS.
Propofol being a lipophilic drug has rapid distribution and elimination times having no cumulative effects after infusion. Propofol has been evaluated in a variety of regimens in ERCP and has been shown to provide superior sedation quality and shorter recovery time [12]. It has been used frequently over the past two decades as sedative agent for endoscopic procedures. Hence, propofol was used in all the 3 study groups as a fixed bolus dose followed by variable intermittent boluses. However, propofol can cause deep sedation or even dangerous side effects needing cardiopulmonary support [2]. Hence, the need for reducing its dose by co administering adjuvants drugs to provide optimal sedation and not compromising on recovery profile. Dexmedetomidine and low dose Ketamine were incorporated in our study by virtue of the favourable recovery profile characters as well as anaesthetic sparing effects [2,7,11].
Dexmedetomidine is a selective alpha-2 agonist with sedative and analgesic properties without respiratory depression. But its use as a sole sedative agent for endoscopic procedures was not found to be effective as per previous studies as it is neither a complete anaesthetic nor a complete analgesic [16]. Hence, we used it in combination with propofol. The dosage implemented in our study is similar to previous studies [7].
Ketamine has made a re-emergence as sedative when used in low dose. Dose related emergence reactions had shelved this versatile NMDA antagonist from routine use in sedation practice. Low dose ketamine defined as <0.5mg/kg has demonstrated potent analgesia, minimal neurophysiologic effects with no clinically significant emergence reactions [11]. The dosage implemented in our study is similar to previous studies [11,17]. Riham et al.,[2], concluded that 1:4 ratio of ketamine and propofol provided better sedation quality than fentanyl/propofol combination with lesser side effects when sedating obese patients undergoing ERCP.
The total amount of propofol consumed was the primary end point of our study. We found a significantly higher dose requirement of propofol in the control group when compared to the dexmedetomidine and ketamine groups, with no significant difference in propofol consumption between the latter two drug groups. Poonam et al., [6] observed a 62.5% reduction in the induction dose of propofol when dexmedetomidine was given along with it. Riham et al., [2], observed significantly lower propofol consumption when 1:4 ketamine/propofol (ketofol) group when compared to fentanyl/propofol group in obese patients undergoing ERCP. Ong et al., [11] also, reported a significant reduction in the total propofol consumption in patients undergoing ERCP when sedated with propofol with cocktail (midazolam 0.5mg, ketamine 15 mg and 6 mg pentazocine) than with propofol alone. The authors concluded that the reduction in total propofol requirement was due to the additive and synergistic effects of the cocktail.
The reasons for using two scoring systems to assess the recovery profile were two fold. The first being that, since we had outpatients as our study volunteers, complete recovery from the anaesthetic drug effects was imperative before discharge. The second being, that although the Modified Aldrette Score being an established objective score for discharge from Post Anaesthesia Care Unit, it does not allow an exact interpretation of patients psychomotor status and is therefore imprecise [18]. Hence, the OAAS score was also incorporated and the time taken to attain the respective discharge scores was analysed. The time to discharge from the recovery room in the ketamine group was 10 ± 4.17 min, which is comparable to the results of Riham et al., [2]. The recovery time of the dexmedetomidine group was significantly prolonged (16.6 min for both scores) when compared with the control (7.5 ± 3.29 min for MAS and 6.68 ± 2.29 min for OAAS) and ketamine group (10 ± 4.17 for MAS and 8.75 ± 3.68 min for OAAS), which could be attributed to the pharmacological profile of dexmedetomidine
Daabis et al., [19], also found that low dose ketamine with propofol (in a 1:4 ratio) had favourable hemodynamic and recovery characteristics when used for procedural sedation in ambulatory settings. Our study concurs the observations on low dose ketamine with propofol as with the other authors. The hemodynamic profile of patients among the groups was comparable to each other, although patients in the dexmedetomidine group had a statistically significant slower heart rate than the other 2 groups, without any clinical significance.
No procedure related complications like intestinal/duct perforation, injury to the upper gastrointestinal structures or bleeding were encountered. No incidence of complications like Bradycardia (heart rate <50 beats/min) [12], hypotension (MAP <20% of baseline) [2], desaturation (SpO2<90%), emergence agitation, aspiration and post procedure nausea and vomiting were documented in our study as the sedation was administered by a trained anesthesiologist, targeting BIS. Although meta analysis [20] showed a trend towards a higher incidence of statistically non significant hypotension with propofol sedation, we did not encounter any significant hypotensive episodes in our patients.
Since, no previous studies have made a direct comparison between dexmedetomidine and ketamine for sedation in outpatient ERCP. Our study shows a comparable profile of the two drugs with respect to propofol consumption and Haemodynamic effects, but with a significantly prolonged recovery time with use of dexmedetomidine with propofol when compared to using propofol alone and propofol with low dose ketamine. The clinical application of this study highlights that low dose ketamine can be considered as a safe alternative to dexmedetomidine for co administration with propofol for patients undergoing ERCP on an outpatient basis.
Consort flow diagram for patients included in the study

| Variable (Mean±SD ) | Control | Dexme-detomidine | Ketamine | p value |
|---|
| Age(years) | 52.7 ± 15.2 | 55.04 ± 13.5 | 44.8 ± 14.5 | 0.063 |
| Weight (kg) | 65.25 ± 17.2 | 58.75 ± 15.2 | 61.5 ± 12.4 | 0.385 |
| MMSE | 30 | 30 | 29.63 ± 0.9 | 0.48 |
| Sr Sodium (mEq/lit) | 134.5 ± 4.7 | 133.3 ± 5.8 | 133.4 ± 4.5 | 0.68 |
Age & weight --- as per Kruskal-Wallis Test; MMSE& Sodium-as per ANOVA
p<0.05 statistically significant
Sex and ASA distribution among the 3 groups
| Variable | Control | Dexmedetomidine | Ketamine | p-value |
|---|
| Sex (Female/Male) | 7/17 | 13/11 | 9/15 | 0.19( chi square test) |
| ASA Status (I/II/III) | 7/15/2 | 8/12/4 | 5/18/1 | 0.962(ANOVA) |
p<0.005 statistically significant
| Variable (Mean±SD ) | Control | Dexmedeto-midine | Ketamine | p-value |
|---|
| Duration of procedure (min) | 29.17 ± 11.57 | 35.21 ± 13.22 | 28.29 ± 11.91 | 1.31 |
| Propofol consumption (mg) | 255.83 ± 114.12 | 162.5 ± 71.7 | 158.33 ± 66.89 | 0.001* |
| Time for attaining Modified Aldrette Score >9 (min) | 7.5 ± 3.29 | 16.6 ± 3.18 | 10 ± 4.17 | < 0.001* |
| Time for attaining Observer Alertness Sedation Score >4 (min) | 6.88 ± 2.47 | 16.67 ± 2.82 | 8.75 ± 3.68 | < 0.001 * |
Kruskall-Wallis test between the 3 groups *p<0.05 = significant
Pre- procedure diagnosis
Chi-Square test; p= 0.295
Endoscopic procedures in 3 groups
Bonferroni Multiple Comparison Test
| Dependent Variable | (I) Group | (J) Group | Mean Difference (I-J) | Std. Error | Sig. |
|---|
| Propofol consumption | Control | Dexmedetomidine | 93.333* | 25.079 | .001 |
| Ketamine | 97.500* | 25.079 | .001 |
| Dexmedetomidine | Control | -93.333* | 25.079 | .001 |
| Ketamine | 4.167 | 25.079 | 1.000 |
| Ketamine | Control | -97.500* | 25.079 | .001 |
| Dexmedetomidine | -4.167 | 25.079 | 1.000 |
| MOD ALDRETE SCORE | Control | Dexmedetomidine | -9.167* | 1.033 | <. 001 |
| Ketamine | -2.500 | 1.033 | .054 |
| Dexmedetomidine | Control | 9.167* | 1.033 | <.001 |
| Ketamine | 6.667* | 1.033 | <.001 |
| Ketamine | Control | 2.500 | 1.033 | .054 |
| Dexmedetomidine | -6.667* | 1.033 | <.001 |
| OAAS | Control | Dexmedetomidine | -9.792* | .877 | <.001 |
| Ketamine | -1.875 | .877 | .108 |
| Dexmedetomidine | Control | 9.792* | .877 | <.001 |
| Ketamine | 7.917* | .877 | <.001 |
| Ketamine | Control | 1.875 | .877 | .108 |
| Dexmedetomidine | -7.917* | .877 | <.001 |
(p< 0.05--- statistically significant)
ANOVA for intergroup Hemodynamic variable comparison
| Variables (Mean±SD ) | Control | Dexmedeto-midine | Ketamine | p-value |
|---|
| Baseline SBP | 142.13 ± 22.2 | 141.54 ± 23.10 | 134.67 ± 19.99 | 0.424 |
| Baseline DBP | 86.67 ± 12.56 | 90.29 ± 16.53 | 85.83 ± 12.71 | 0.509 |
| Baseline SpO2 | 99.42 ± 1.13 | 99.63 ± 0.87 | 99.29 ± 0.85 | 0.486 |
| Baseline BIS | 97.42 ± 1.64 | 97.21 ± 1.31 | 96.79 ± 2.28 | 0.473 |
p<0.05 -significant
Heart rate distribution among the 3 groups

Distribution of mean arterial pressure among the 3 groups

Mixed Model Analysis comparing heart rates and Mean Arterial Pressures
| Group | Heart Rate Mean ± Std error | p-value | Mean Arterial Pressure (Mean ± Std error) | p-value |
|---|
| Control | 105.290 ± 2.711 | 0.271 | 100.16 ± 2.13 | 0.35 |
| Dexmedetomidine | 81.180 ± 2.643 | <0.001 | 97.39 ± 2.16 | 0.45 |
| Ketamine | 102.347 ± 2.719 | 0.060 | 107 ± 2.20 | 0.20 |
p<0.005 -statistically significant
Mixed model analysis for means Heart rate difference between 3 groups
| (I) Group | (J) Group | Mean Difference (I-J) | Std. Error | p- value |
|---|
| Control | Dexmedetomidine | 24.110* | 3.787 | <.001 |
| Ketamine | 2.943 | 3.840 | .446 |
| Dexmedetomidine | Control | -24.110* | 3.787 | <.001 |
| Ketamine | 21.167* | 3.792 | <.001 |
| Ketamine | Control | -2.943 | 3.840 | .446 |
| Dexmedetomidine | 21.167* | 3.792 | <.001 |
p<0.05 -significant
Limitations
Our study has some limitations. We did not evaluate the possibility of intra procedural awareness as we had monitored the depth of anaesthesia using BIS. However, the occurrence of awareness cannot be ruled out. Also, influence of dexmedetomidine and ketamine on BIS values was not independently considered. Consumption of alcohol may have been a confounding factor among the groups due to its interaction with the anaesthetic drugs used.
Conclusion
Low dose ketamine, with background propofol boluses resulted in a lesser propofol consumption, with earlier recovery and favourable hemodynamic profile when compared with dexmedetomidine and control group in outpatients undergoing ERCP.
Age & weight --- as per Kruskal-Wallis Test; MMSE& Sodium-as per ANOVA p<0.05 statistically significant
p<0.005 statistically significant
Kruskall-Wallis test between the 3 groups *p<0.05 = significant
(p< 0.05--- statistically significant)
p<0.05 -significant
p<0.005 -statistically significant
p<0.05 -significant
[1]. S Von Delius, H Salletmaier, A Meining, S Wagenpfeil, D Saur, M Bajbouj, Bispectral index monitoring of midazolam and propofol sedation during endoscopic retrograde cholangio pancreatography: a randomized clinical trial (the EndoBIS study)Endoscopy 2012 44:258-64. [Google Scholar]
[2]. R Hasanein, W El-Sayed, Ketamine / Propofol versus fentanyl / propofol for sedating obese patients undergoing endoscopic retrograde cholangiopancreaticography (ERCP)Egyptian Journal of Anaesthesia 2013 29:201-11. [Google Scholar]
[3]. JW Johansen, PS Sebel, Development and Clinical Application of Electroencephalographic Bispectrum MonitoringAnesthesiology 2000 93:1336-44. [Google Scholar]
[4]. LP Fabbri, M Nucera, M Marsili, M Al Malyan, C Becchi, Ketamine, propofol and low dose remifentanil for ERCP outside the operating room: Is ketamine only a “rescue drug”?Med Sci Monit 2012 18(9):575-80. [Google Scholar]
[5]. D Garewal, S Powell, SJ Milan, J Nordmeyer, P Waikar, Sedative techniques for endoscopic retrograde cholangio pancreatographyCochrane Database of Systematic Reviews 2012 6(7274)doi:10.1002/14651858.CD007274.pub2 [Google Scholar]
[6]. PS Ghodki, SK Thombre, SP Sardesai, KD Harnagle, Dexmedetomidine as an adjuvant in laparoscopic surgery. An observational study using entropy monitoringJ Anaesthesiol Clin Pharmacol 2012 28:334-38. [Google Scholar]
[7]. HW Shin, HN Yoo, DH Kim, H Lee, HJ Shin, HW Lee, Pre-anaesthetic Dexmedetomidine 1μg/kg single infusion is a simple, easy and economic adjuvant for general anaesthesiaKorean J Anesthesiol 2013 65(2):114-20. [Google Scholar]
[8]. D Ozcengez, H Unlugenc, KY Gunes, F Karacaer, The effect of dexmedetomidine on bispectral index monitoring in childrenMiddle East J Anesth 2012 21:613-18. [Google Scholar]
[9]. JA Aldrete, The post anaesthesia recovery score revisitedJ Clin Anesth 1995 7:89-91. [Google Scholar]
[10]. RE Anderson, JG Jakobsson, Cerebral state index: comparison between pairwise registrations from the left and the right sides of the brain.Br J Anaesth 2006 97(3):347-50. [Google Scholar]
[11]. WC Ong, D Santosh, S Lakhtakia, DN Reddy, Randomised Controlled Trial on use of propofol with midazolam, ketamine, and pentazocine “sedato-analgesic cocktail” for sedation during ERCPEndoscopy 2007 39:807-12. [Google Scholar]
[12]. SY Jang, HG Park, MK Jung, Bispectral Index Monitoring as an adjunct to Nurse administered Combined sedation during Endoscopic retrograde cholangiopancreatographyWorld J Gastroenterol 2012 18(43):6284-89. [Google Scholar]
[13]. DR Lichtenstein, S Jagannath, TH Baron, Sedation and anaesthesia in G I endoscopyGastrointest Endosc. 2008 68:205-16. [Google Scholar]
[14]. T Wehrmann, J Grotkamp, N Stergiou, A Riphaus, A Kluge, B Lembcke, Electroencephalogram monitoring facilitates sedation with propofol for routine ERCP A randomized Controlled Trail. Gastrointset Endosc 2002 56:817-24. [Google Scholar]
[15]. SS Liu, Effects of Bispectral Index monitoring on Ambulatory Anaesthesia: A meta-analysis of randomized controlled trails and cost analysisAnesthesiology 2004 101:311-15. [Google Scholar]
[16]. BJ Ko, JH Jang, JW Park, SC Park, R Lee, SR Choi, Procedural sedation with Dexmedetomidine for pediatric endoscopic retrograde cholangiopancreatography guided stone extractionKorean J Anesthesiol 2012 63(6):567-68. [Google Scholar]
[17]. A Kirberg, R Sagredo, G Montalva, E Flores, Ketamine for paediatric endoscopic procedures and as a sedation, complement for adult patientGastrointest Endosc 2005 61:501-02. [Google Scholar]
[18]. J Willey, JJ Vargo, JT Connor, Quantitative assessment of psychomotor recovery after sedation and analgesia for outpatient EJDGastrointest Endosc 2002 56:810-16. [Google Scholar]
[19]. M Daabis, M Elsherbiny, R Alotibi, Assessment of different concentration of ketofol in procedural operationBritish Medical Journal 2009 2(1):27-31. [Google Scholar]
[20]. LL Bo, Y Bai, JJ Bian, PS Wen, JB Li, XM Deng, Propofol versus traditional Sedative agents for endoscopic retrograde cholangiography: A meta- analysisWorld J Gastroenterol 2011 17(30):3538-43. [Google Scholar]