Association between Experimental Bacterial Meningitis and Periapical Lesion
Tatiana Barichello1, Soraia Netto2, Renan Antonio Ceretta3, Jaqueline S. Generoso4, Lutiana R. Simões5, Patrícia Ávila Ribeiro6, Josiane Budni7, João Quevedo8
1 Laboratório de Microbiologia Experimental, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
2 Laboratório de Microbiologia Experimental, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
3 Laboratório de Microbiologia Experimental, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
4 Laboratório de Microbiologia Experimental, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
5 Laboratório de Microbiologia Experimental, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
6 Laboratório de Microbiologia Experimental, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
7 Laboratório de Neurociências, Programa de Pós-Graduação em Ciências da Saúde, Unidade Acadêmica de Ciências da Saúde, Universidade do Extremo Sul Catarinense, 888806-000, Criciúma, SC, Brazil.
8 Center for Experimental Models in Psychiatry, Department of Psychiatry and Behavioral Sciences, The University of Texas Medical School at Houston, 77030, Houston, TX, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Tatiana Barichello, PhD - Laboratório de Microbiologia Experimental, PPGCS, UNASAU, Universidade do Extremo Sul Catarinense, 88806-000 Criciúma, SC, Brazil.
E-mail: tba@unesc.net
Introduction
Mortality and morbidity from bacterial meningitis in African adults is significantly higher than those in better resourced settings. At the same time, the periodontal diseases are highly prevalent and can affect up to 90% of the population. Dental caries in Uganda was recorded in 40% and 62.5% of the children and adults, respectively. We hypothesize that pneumococcal meningitis could interfere in the development of periapical lesion. The aim of this study was to evaluate periapical lesion in Wistar rats subjected to pneumococcal meningitis.
Materials and Methods
The animals were divided in control, control/periapical lesion, meningitis, and meningitis/periapical lesion groups. The surgical exposure of molars and the infection of the dental pulp were from the oral environment. Pulp necrosis was induced on the left mandibular first molars during adulthood. Dental pulps were exposed by drilling cavities on the central portion of the occlusal surface with a 1011 HL round bur in high speed to a depth nearly equal to the bur diameter. Animals were subjected to behavioral task and evaluation of the size of periodontal ligament. Data from periodontal ligament space and open field task were reported as mean ± SEM and analysed by Two-way ANOVA and paired Student’s t-test, respectively.
Results and Conclusion
Meningitis/periapical increased the periodontal ligament space by 61% when compared with control/periapical. In the open-field task, there were no differences in the number of crossings and rearing movements between training and test session in meningitis and periapical lesion groups demonstrating habituation memory impairment. Bacterial meningitis and periapical lesion may play an important role in development of cognitive impairment.
Memory, Pneumococcal meningitis, Periodontal ligament
Introduction
Mortality from bacterial meningitis in African adults is significantly higher than those in better resourced settings. In sub-Saharan Africa, a study with 715 episodes of bacterial meningitis, the mortality rate was 45% at day 10 and 54% at day 40 [1]. At the same time, the periodontal diseases are highly prevalent and can affect up to 90% of the worldwide population [2]. Dental caries in Uganda was recorded in 40% and 62.5% of the children and adults, respectively [3]. Thus, could have an association between neuronal infection and periapical lesion. In Africa, the most common pathogens responsible for bacterial meningitis were Streptococcus pneumoniae (84%) and Neisseria meningitidis (4%) [1]. Pneumococcal meningitis infection is a life-threatening condition with high mortality and neurological sequelae [4,5]. The bacterial compounds are highly immunogenic and might facilitate an increased inflammatory response in the host [6]. These pro-inflammatory mediators are expressed by various host cells in response to bacterial infection and are effective stimulators of bone resorption [7]. Bone loss also was demonstrated in Wistar rats subjected to bilateral hippocampus lesion, which developed significantly more destruction of the periodontium than their controls [8]. Exposure of molars and infection of the dental pulp from the oral environment leads to outcome in the expansion of periapical lesions and damage of bone [7]. Cells that express bone-resorptive cytokines are present immediately after pulp exposure, which supports the hypothesis that these mediators play a key role in pulpal and periapical dental pathogenesis, including the concomitant bone destruction [9]. We hypothesize that pneumococcal meningitis might interfere in the development of periapical lesion. For this reason, the aim of the present study was to evaluate periapical lesion in Wistar rats subjected to pneumococcal meningitis.
Materials and Methods
Infecting Organism
Streptococcus pneumoniae (serotype 3) was cultured in Todd Hewitt broth, and it was diluted in fresh medium. This culture was centrifuged for 10 min at 5,000×g and resuspended in sterile saline at a concentration of 5x109 cfu/ml [10].
Animal Model of meningitis
Male Wistar rats (250-300 g body weight) from our breeding colony were used for the experiments. This experiment was approved by the Animal Care and Experimentation Committee of UNESC, Brazil, and was followed in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996. The animals were anesthetized with an intraperitoneal administration of ketamine (6.6 mg/kg) and xylazine (0.3 mg/kg) [11]. Rats underwent a cisterna magna tap with a 23-gauge needle and received either 10 μl of artificial cerebro spinal fluid (CSF) as a placebo or 10 μl of the S. pneumoniae suspension. Immediately after induction, the animals received fluid replacement and were then returned to their cages [11,12]. Meningitis was documented by a quantitative culture of 5 μl of CSF obtained by puncture of the cisterna magna.
Animal model of periapical lesion
Wistar rats were anesthetizes by an intraperitoneal administration of ketamine (6.6 mg/kg), xylazine (0.3 mg/kg), and acepromazine (0.16 mg/kg) [13]. The surgical exposure of molars and the infection of the dental pulp were from the oral environment, occurring reproducible results in the development of periapical lesions and the destruction of bone. Pulp necrosis was induced on the left mandibular first molars during adulthood. Dental pulps were exposed by drilling cavities on the central portion of the occlusal surface with a 1011 HL round bur in high speed (KG Sorensen, Cotia, SP, Brazil) to a depth nearly equal to the bur diameter (1 mm) [14].
Organization of the Experimental Groups
The animals were divided into four groups: control (n = 10), control/periapical lesion (n = 10), meningitis (n = 10), and meningitis/periapical lesion (n = 10). Three weeks after meningitis induction and periapical lesion the animals were subjected to behavioural task.
Behavioral task (Open field task)
Both locomotor and exploratory activities were assessed in an open-field apparatus to evaluate. This apparatus consist of a 40 x 60 cm open-field and with 50 cm high walls of brown plywood and a front glass wall. Black lines divide the floor of the open-field into nine rectangles. Animals were gently placed on the left rear quadrant and were left to explore the arena for 5 min. The number of crossings (number of times that the animals crossed the black lines, locomotor activity) and rearing movements (the exploration behavior observed in rats subjected to a new environment) were measured. The same researcher, who was blind to group treatment, performed all behavioral testing by manual analyses [15].
Radiographic evaluation of periapical lesion
The X-ray cylinder was fitted to form a perpendicular angle with the buccal surface of the first molar. A focal distance of 30 cm was observed. The X-ray unit operated at 7 mA at 70 kVp, with a size 2 phosphor plate [14] (Dabi Atlante, São Paulo) and exposure time of 0.2 seconds. Digital X-ray system was used to capture images scanned (Vista Scan Durr, São Paulo) at the resolution of 1000 dpi and saved in JPEG format.
Statistical Analysis
Data from periodontal ligament space were reported as mean ± SEM and analysed by Two-way ANOVA. Data from the habituation to an open field task were reported as mean ± SEM, and it was analysed by the paired Student’s t-test to compare training with test session and ANOVA post-hoc Tukey to compare differences in the number of crossings and rearing movements among groups. P-values <0.05 were considered statistically significant. All analyses were performed using the Statistical Package for the Social Science (SPSS) software version 20.0.
Results
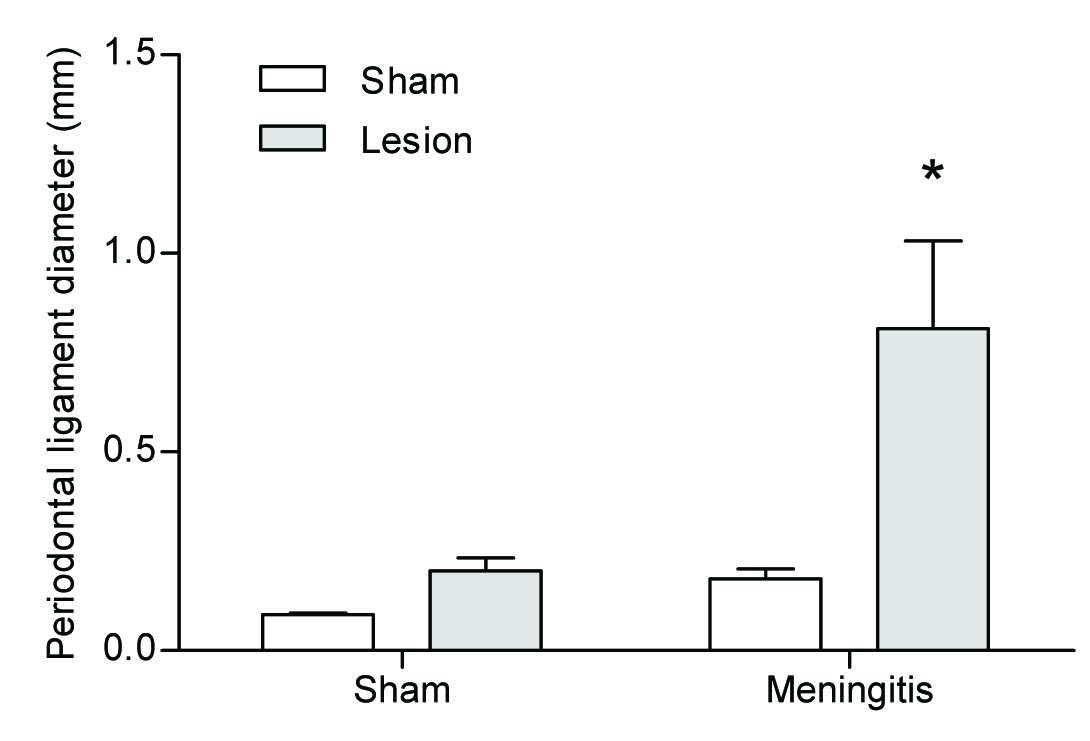
The measure of the periodontal ligament space three weeks after lesion was: control (0.09 ± 0.013), control/periapical lesion (0.20 ± 0.09), meningitis (0.18 ± 0.08), and meningitis/periapical lesion (0.81 ± 0.07). We demonstrated the effects of pneumococcal meningitis on periodontal ligament space. Meningitis/periapical lesion group was considered statistically different when compared with control/periapical lesion group (F1,35 = 9.09; p = 0.005). There was an interaction of pneumococcal meningitis on periodontal ligament space. We demonstrated that meningitis/periapical lesion group increased the periodontal ligament space by 61% when compared with control/periapical lesion group (F1,35 = 4.987; p = 0.032) [Table/Fig-1].
Measure of the periodontal ligament space three weeks after lesion. The animals were separated in four groups: sham; sham/periapical lesion; meningitis and meningitis/periapical lesion
In all comparisons, p<0.05 indicated statistical significance. Data from periodontal ligament space were reported as mean ± SEM and analysed by Two-way ANOVA
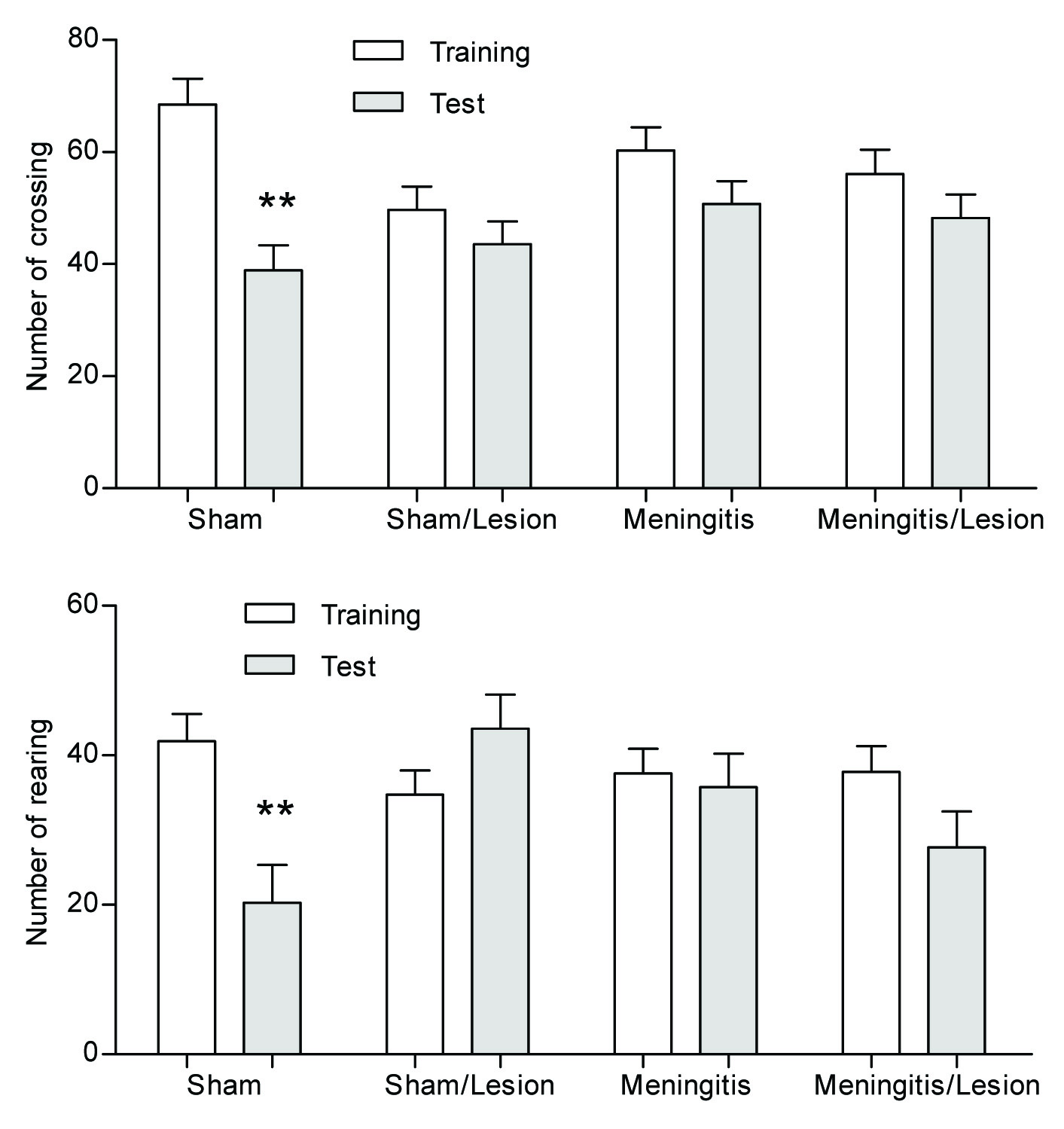
In the Open-Field task, we evaluated the influence of periapical lesion on habituation memory three weeks after lesion. There were no differences in the number of crossings and rearing movements among groups in the habituation to the Open-Field training session, demonstrating no difference in motor and exploratory activity among groups. In the test session, there was a significant reduction in both crossings and rearing in the control group compared with the training session, demonstrating habituation memory (p<0.05). However, the control/periapical; meningitis and meningitis/periapical lesion groups showed no difference in motor and exploratory activity between training and test sessions, demonstrating habituation memory impairment in these groups, [Table/Fig-2].
Influence of periapical lesion on habituation memory three weeks after lesion through Open Field task. The animals were separated in four groups: sham; sham/periapical lesion; meningitis and meningitis/periapical lesion. Data are reported as mean ± SEM, n = 10 per group, and were analysed by the paired Student’s t-test and ANOVA post-hoc Tukey
In all comparisons, p<0.01 indicated statistical significance
In [Table/Fig-3], radiograph showing well-defined size of periapical lesion: A) control, B) control/periapical lesion, C) meningitis, and D) meningitis/periapical lesion.
Radiograph showing well-defined size of periapical lesion. The animals were separated in four groups: A) sham; B) sham/periapical lesion; C) meningitis and D) meningitis/periapical lesion

Discussion
In the present study, we evaluated the influence of pneumococcal meningitis on periapical lesion in an experimental rat model. There was an interaction of pneumococcal meningitis on periodontal ligament size by 61% when compared with control group of periapical lesion. Other studies have documented that the hippocampal lesioned rats developed more destruction of the periodontium [8], maternal periapical lesion was associated with brain inflammation in rat pups [16], and inflammatory lesions of the tooth pulp induced changes in brainstem neurons of the rats [17]. We also verified that animals subjected to periapical lesion and animals subjected to periapical lesion concomitant with pneumococcal meningitis both had impairment of memory three weeks after lesion and meningitis induction. In previous studies, animals subjected to pneumococcal meningitis showed memory and learning deficits, anxiety-like and depressive-like behavior ten days after induction [18,19]. The host immune response, the production of cytokines and chemokines, and leukocyte migration represent the first line of defense in response to bacterial infection [20]. Increased TNF-α, IL-1β and IL-6 concentrations in the CSF were found in bacterial meningitis [21]. In addition, after pneumococcal meningitis, TNF-α, IL-1β, IL-6 and CINC-1 were produced mainly in the first 6 to 24 h in the cortex and TNF-α and CINC-1 in the hippocampus [22]. Oxidative stress also was formed at 24 h and 48 h after induction [23]. The same cytokines, TNF-α, IL-1β and IL-6 are commonly associated with oral infections involving periodontal and periapical lesion [16], periapical abscesses in pregnant rats also resulted in increased of the TNF-α, IL-1β and IL-6 in brain of the rat pups [16]. Although the hippocampus is not exposed to bacteria or infiltrating leukocytes directly, it is surrounded by an interstitial fluid, which is contiguous with the cerebral spinal fluid, permitting the secreted bacterial toxins and cytokines, chemokines to diffuse into the brain parenchyma [4]. In addition, life stress, anxiety, depression, can induce changes in regulatory mechanisms within the brain involved in immune regulation, and thereby alter immune responses and influence the susceptibility or resistance to inflammatory disorders [24]. We showed that depressive-like behavior ten days after pneumococcal meningitis induction was reversed by treatment with the antidepressant imipramine [19]. In addition, the olfactory bulbectomy, a model of depression in rats enhanced susceptibility to perioodontitis [24], and periodontal disease, multiple dental caries, and periapical pathology were associated with cerebral abscess [25].
Conclusion
We believe that these findings suggest that the pneumococcal meningitis may play an important role in progression of periapical lesion.
[1]. Wall EC, Cartwright K, Scarborough M, Ajdukiewicz KM, Goodson P, Mwambene J, High mortality amongst adolescents and adults with bacterial meningitis in sub-Saharan Africa: an analysis of 715 cases from MalawiPloS one 2013 8:e69783 [Google Scholar]
[2]. Pihlstrom BL, Michalowicz BS, Johnson NW, Periodontal diseasesLancet 2005 366:1809-20. [Google Scholar]
[3]. Muwazi LM, Rwenyonyi CM, Tirwomwe FJ, Ssali C, Kasangaki A, Nkamba ME, Prevalence of oral diseases/conditions in UgandaAfrican health sciences 2005 5:227-33. [Google Scholar]
[4]. Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D, Pathogenesis and pathophysiology of pneumococcal meningitisClin Microbiol Rev 2011 24:557-91. [Google Scholar]
[5]. Nau R, Soto A, Bruck W, Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitisJ Neuropathol Exp Neurol 1999 58:265-74. [Google Scholar]
[6]. Barichello T, Generoso JS, Simoes LR, Elias SG, Quevedo J, Role of oxidative stress in the pathophysiology of pneumococcal meningitisOxidative medicine and cellular longevity 2013 2013:371465 [Google Scholar]
[7]. Stashenko P, Wang CY, Tani-Ishii N, Yu SM, Pathogenesis of induced rat periapical lesionsOral Surg Oral Med Oral Pathol 1994 78:494-502. [Google Scholar]
[8]. Breivik T, Thrane PS, Gjermo P, Cools A, Myhrer T, Effects of hippocampal lesioning on experimental periodontitis in Wistar ratsJournal of periodontal research 2002 37:360-65. [Google Scholar]
[9]. Tani-Ishii N, Wang CY, Stashenko P, Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposureOral microbiology and immunology 1995 10:213-39. [Google Scholar]
[10]. Barichello T, Pereira JS, Savi GD, Generoso JS, Cipriano AL, Silvestre C, A kinetic study of the cytokine/chemokines levels and disruption of blood-brain barrier in infant rats after pneumococcal meningitisJ Neuroimmunol 2011 233:12-7. [Google Scholar]
[11]. Barichello T, Generoso JS, Silvestre C, Costa CS, Carrodore MM, Cipriano AL, Circulating concentrations, cerebral output of the CINC-1 and blood-brain barrier disruption in Wistar rats after pneumococcal meningitis inductionEuropean journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 2012 [Google Scholar]
[12]. Irazuzta JE, Pretzlaff RK, Zingarelli B, Xue V, Zemlan F, Modulation of nuclear factor-kappaB activation and decreased markers of neurological injury associated with hypothermic therapy in experimental bacterial meningitisCrit Care Med 2002 30:2553-59. [Google Scholar]
[13]. Barichello T, Generoso JS, Silvestre C, Costa CS, Carrodore MM, Cipriano AL, Circulating concentrations, cerebral output of the CINC-1 and blood-brain barrier disruption in Wistar rats after pneumococcal meningitis inductionEur J Clin Microbiol Infect Dis 2012 31:2005-09. [Google Scholar]
[14]. Scarparo RK, Dondoni L, Bottcher DE, Grecca FS, Rockenbach MI, Batista EL, Jr, Response to intracanal medication in immature teeth with pulp necrosis: an experimental model in rat molarsJ Endod 2011 37:1069-73. [Google Scholar]
[15]. Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the ratLearn Mem 2000 7:333-40. [Google Scholar]
[16]. Bain JL, Lester SR, Henry WD, Pongetti JL, Blackman ME, Johnson RB, Association between maternal periapical lesions and brain inflammation in rat pupsArchives of oral biology 2013 58:266-71. [Google Scholar]
[17]. Torneck CD, Kwan CL, Hu JW, Inflammatory lesions of the tooth pulp induce changes in brainstem neurons of the rat trigeminal subnucleus oralisJournal of dental research 1996 75:553-61. [Google Scholar]
[18]. Barichello T, Silva GZ, Generoso JS, Savi GD, Michelon CM, Feier G, Time-dependent behavioral recovery after pneumococcal meningitis in ratsJ Neural Transm 2010 117:819-26. [Google Scholar]
[19]. Barichello T, Milioli G, Generoso JS, Cipriano AL, Costa CS, Moreira AP, Imipramine reverses depressive-like parameters in pneumococcal meningitis survivor ratsJournal of neural transmission 2012 119:653-60. [Google Scholar]
[20]. Scheld WM, Koedel U, Nathan B, Pfister HW, Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injuryJ Infect Dis 2002 186(Suppl 2):S225-33. [Google Scholar]
[21]. Ostergaard C, Brandt C, Konradsen HB, Samuelsson S, Differences in survival, brain damage, and cerebrospinal fluid cytokine kinetics due to meningitis caused by 3 different Streptococcus pneumoniae serotypes: evaluation in humans and in 2 experimental modelsJ Infect Dis 2004 190:1212-20. [Google Scholar]
[22]. Barichello T, dos Santos I, Savi GD, Simoes LR, Silvestre T, Comim CM, TNF-alpha, IL-1beta, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniaeJ Neuroimmunol 2010 221:42-45. [Google Scholar]
[23]. Barichello T, Savi GD, Silva GZ, Generoso JS, Bellettini G, Vuolo F, Antibiotic therapy prevents, in part, the oxidative stress in the rat brain after meningitis induced by Streptococcus pneumoniaeNeurosci Lett 2010 478:93-96. [Google Scholar]
[24]. Breivik T, Gundersen Y, Myhrer T, Fonnum F, Osmundsen H, Murison R, Enhanced susceptibility to periodontitis in an animal model of depression: reversed by chronic treatment with the anti-depressant tianeptineJ Clin Periodontol 2006 33:469-77. [Google Scholar]
[25]. Mylonas AI, Tzerbos FH, Mihalaki M, Rologis D, Boutsikakis I, Cerebral abscess of odontogenic originJournal of cranio-maxillo-facial surgery: official publication of the European Association for Cranio-Maxillo-Facial Surgery 2007 35:63-67. [Google Scholar]