Introduction
Several investigations have been made to determine the level of alpha-synuclein in the peripheral blood of Parkinson’s disease patients, but the results were very contradictory and inconclusive. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) was also found to be involved in Parkinson’s disease, but there is a lack of studies investigating NF-κB in Parkinson’s disease patients. As far as our knowledge goes, no study reported the level of NF-κB in serum of Parkinson’s disease patients. In this context, present study was designed to make a baseline study in order to evaluate the levels of total alpha-synuclein and NF-κB in serum of Parkinson’s disease patients.
Materials and Methods
Serum samples were collected from Parkinson’s disease patients (n=97) and healthy controls (n=97). Their levels were determined by using Enzyme linked immunosorbent assay.
Results
The levels of total alpha-synuclein (patients=5.79±2.24 ng/ml, controls=6.20±1.61 ng/ml; p=0.14) and NF-κB (patients=1.38±0.99 ng/ml, controls=1.65±1.00 ng/ml; p=0.71) were lower in Parkinson’s disease patients than controls, but the differences were not statistically significant. This difference was also failed to reach significance between males (alpha-synuclein (p)=0.70, NF-κB (p)=0.84) and females (alpha-synuclein (p)=0.06, NF-κB (p)=0.77) in both the groups as well as within the groups. The levels of total alpha-synuclein and NF-κB were also not associated with Parkinson’s disease severity (alpha-synuclein (p) = 0.84, NF-κB (p) = 0.73).
Conclusion
A non-significant difference in the levels of total alpha-synuclein and NF-κB between Parkinson’s disease patients and controls suggested that these are not valuable biomarkers for Parkinson’s disease, more specifically in Indian population.
Alpha-synuclein, Biomarkers, Enzyme linked immunosorbent assay, NF-κB, Parkinson’s disease
Introduction
Alpha-synuclein is a pre-synaptic protein whose misfolding and dysfunction contribute to the pathogenesis of Parkinson’s disease (PD) [1]. Though alpha-synuclein is abundantly expressed in the human brain, neuronal release causes its presence in extracellular biological fluids, such as cerebrospinal fluid (CSF), plasma and serum as evidenced by previous studies [1–3]. Alpha-synuclein is one of the major constituents of cytoplasmic Lewy bodies [4], might be a useful biomarker for PD, but researches have shown different results regarding alpha-synuclein as a PD biomarker. Some studies reported a higher level or lower level of alpha-synuclein and some studies not. For example, Lee et al., [1], Duran et al., [5] and El-Agnaf et al., [6] found significantly higher levels of alpha-synuclein in PD patients when compared with controls. Contrary to these studies, significantly lower levels of alpha-synuclein was also observed in PD patients than age-matched controls [7,8]. However, Caranci et al., found no significant difference in the level of alpha-synuclein between PD and healthy controls [9].
The variation in the results among these studies might have resulted from several factors, including detection method, reagents used in the assays, different forms of alpha-synuclein, sample size, inadequate controls with confounding factors, and the population studied. So, there is a need for comparative studies detecting alpha-synuclein level in subjects from different countries to prove alpha-synuclein as a clinically useful biomarker for PD. Possibly, our study could contribute to the controversy of alpha-synuclein in PD patients.
Apart from alpha-synuclein, NF-κB, a transcription factor that controls genes encoding for pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors and inducible enzymes [10] suggested its association with PD. However, very few studies are available investigating NF-κB in PD patients. By the use of immunohistochemistry, Hunot et al., found a seventy fold increase in immunoreactive NF-κB in proportion with dopaminergic neurons in PD brain than controls in French population [11]. In support of this, Mogi et al., also found higher level of NF-κB in the nigrostriatal dopaminergic regions in parkinsonian patients than controls in Japanese population [12]. Significant difference in the level of NF-κB was observed in peripheral blood mononuclear cells (PBMC) from PD patients than healthy controls [10]. As far as our knowledge goes, the level of NF-κB has not been investigated in the serum of PD patients. In the earlier context, this study was designed to make a baseline study to evaluate the levels of total alpha-synuclein and NF-κB in serum of PD patients by using enzyme linked immunosorbent assay (ELISA).
Materials and Methods
Subjects
The present study included 97 patients with idiopathic PD and equal number of controls. Patients were enrolled at the outpatient clinic of the Department of Neurology, King George’s Medical University, Lucknow, India. Diagnosis of PD was made according to United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnosis Criteria [13] and the clinical stages of PD were evaluated according to the classification of Hoehn and Yahr (2001) [14]. Patients with secondary Parkinson’s disease were excluded from the study. Healthy controls were recruited from the local community. Controls, having history of any neurological disorder were excluded from the study. The absence of disease was confirmed by clinical history and physical examination.
All the study procedures were approved by the institutional ethics committee. Informed consent was obtained from all the study subjects. In few PD patients, who were not in the state of giving consent, approval was sought by their guardians.
Serum collection and ELISA
Five ml of blood sample was drawn carefully and allowed to clot at room temperature. Each sample was centrifuged at 2400 g rpm for 10 minutes to separate the serum. All the serum samples were stored at -80oC until assayed. The levels of total alpha-synuclein and NF-κB in serum were measured using Human α-synuclein ELISA (Invitrogen, Catalog#KHB0061) [3] and Human NF-κB enzyme-linked immunosorbent assay (Uscn, Catalog# E91824Hu) kits, respectively according to the directions of the manufacturer’s. All the samples were tested in duplicate. The optical density of the samples was determined at 450 nm with iMark microplate absorbance reader.
Statistical Analysis
All the analysis was carried out using SPSS 16.0 version (Chicago, Inc. USA). The results are presented in mean±SD and percentage. The dichotomous variables were compared by using Chi-square test and continuous variables were compared with Mann-Whitney U test. Spearman correlation was used to see the association between PD and the levels of total alpha-synuclein and NF-κB. The p-value was set at <0.05 as significant.
Results
All the demographic details are summarized in [Table/Fig-1]. No significant differences were found in the levels of total alpha-synuclein (PD=5.79±2.24 ng/ml, controls=6.20±1.61 ng/ml; p=0.14) and NF-κB (PD=1.38±0.99 ng/ml, controls=1.65±1.00 ng/ml; p=0.71) between PD patients and controls. The levels of total alpha-synuclein and NF-κB exhibited no significant difference between males (alpha-synuclein (p)=0.70, NF-κB (p)=0.84) and females (alpha-synuclein (p)=0.06, NF-κB (p)=0.77) in both the groups as well as within the groups i.e., males vs. females (alpha-synuclein: PD (p)=0.32, controls (p)=0.40; NF-κB: PD (p)=0.06, controls (p)=0.40). A poor correlation was found between the age and level of total alpha-synuclein (PD: r=0.09, p=0.36; controls: r=0.04, p=0.75) in both the groups. Similar observation was also found between age and level of NF-κB (PD: r=-0.05, p=0.60; controls: r=-0.20, p=0.07) in both the groups. The levels of total alpha-synuclein and NF-κB was also poorly correlated with age at PD onset (alpha-synuclein: r=0.10, p=0.36; NF-κB: r=-0.03, p=0.77) and disease duration (alpha-synuclein: r=-0.02, p=0.90; NF-κB: r=-0.02, p=0.82). However, levels of total alpha-synuclein and NF-κB were further analysed in detail with respect to different categories of age, age at PD onset and disease duration in [Table/Fig-2,3].
Demographics of study subjects
| Characteristics | PD (n=97) | Controls (n=97) | p-value |
|---|
| Age (years) | 56.34±9.58 | 53.30±8.33 | 0.24 |
| Age (years) at PD onset | 52.59±11.18 | - | - |
| Weight (Kg) | 53.98±10.70 | 56.40±7.45 | 0.07 |
| Duration of PD (months) | 37.64±32.93 | - | - |
| Sex (M/ F) | 69/28 | 68/29 | 0.87 |
PD= Parkinson’s disease, n= Number
Values are expressed as mean±SD
Mean level of total alpha-synuclein (ng/ml) in study subjects
| Characteristics | PD (n=97) | Controls (n=97) | p-value |
|---|
| Alpha-synuclein | 5.79±2.24 | 6.20±1.61 | 0.14 |
| Gender |
| Male | 5.89±2.26 (n=69) | 6.11±1.76 (n=68) | 0.70 |
| Female | 5.53±2.19 (n=28) | 6.41±1.18 (n=29) | 0.06 |
| p-value | 0.32 | 0.40 | |
| Age (years) |
| <55 | 5.78±2.01 (n=34) | 6.12±1.61 (n=51) | 0.384 |
| >55 | 5.79±2.36 (n=63) | 6.28±1.62 (n=46) | 0.234 |
| p-value | 0.970 | 0.636 | |
| Age at PD onset (years) |
| <55 | 5.66±2.22 (n=48) | - | - |
| >55 | 5.91±2.27 (n=49) | - | - |
| p-value | 0.582 | | |
| Disease duration (months) |
| <60 | 5.98±2.00 (n=72) | - | - |
| >60 | 5.25±2.78 (n=25) | - | - |
| p-value | 0.161 | | |
PD= Parkinson’s disease, n= Number
Values are expressed as mean±SD
Mean level of NF-κB (ng/ml) in study subjects
| Characteristics | PD (n=97) | Controls (n=97) | p-value |
|---|
| NF-κB | 1.38±0.99 | 1.65±1.00 | 0.71 |
| Gender |
| Male | 1.50±1.09 (n=69) | 1.80±1.69 (n=68) | 0.84 |
| Female | 1.08±0.60 (n=28) | 1.29±1.14 (n=29) | 0.77 |
| p-value | 0.06 | 0.40 | |
| Age (years) |
| <55 | 1.42±0.97 (n=34) | 1.94±1.72 (n=51) | 0.104 |
| >55 | 1.36±1.01 (n=63) | 1.32±1.30 (n=46) | 0.858 |
| p-value | 0.841 | 0.051 | |
| Age at PD onset (years) |
| <55 | 1.39±1.02 (n=48) | - | - |
| >55 | 1.38±0.97 (n=49) | - | - |
| p-value | 0.977 | | |
| Disease duration (months) |
| <60 | 1.36±0.939 (n=72) | - | - |
| >60 | 1.43±1.115 (n=25) | - | - |
| p-value | 0.776 | | |
PD= Parkinson’s disease, n= Number
Values are expressed as mean±SD
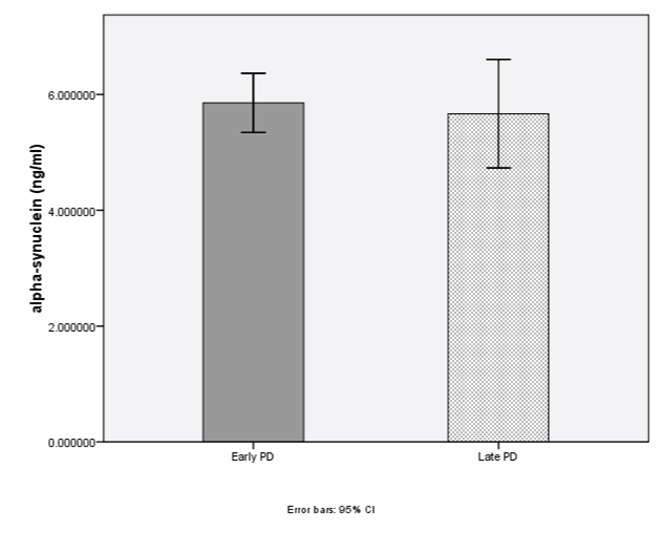
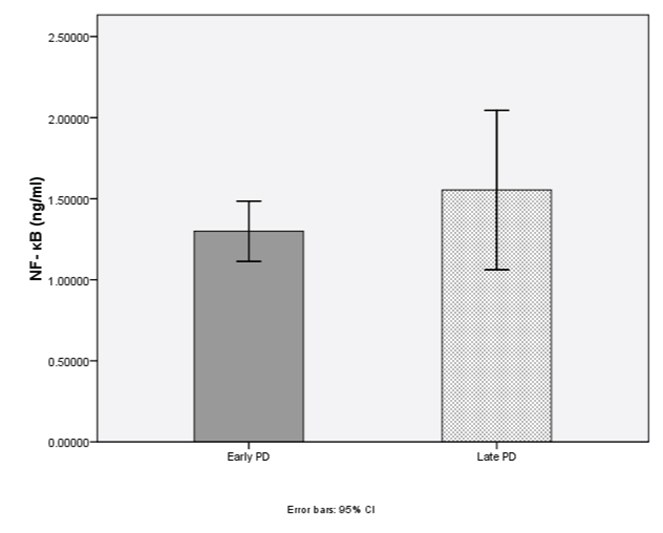
According to disease severity, the levels of total alpha-synuclein (early PD=5.85±2.06 ng/ml, late PD=5.66±2.60 ng/ml; p=0.84) and NF-κB (early PD=1.29±0.74 ng/ml, late PD=1.55±1.36 ng/ml; p=0.73) were not significantly different [Table/Fig-4,5].
Level of alpha-synuclein (p=0.84) in early PD and late PD
Level of NF-κB (p=0.73) in early PD and late PD
The studies from various countries regarding the level of total alpha-synuclein and its different forms including, oligomeric, phosphorylated, oligo-phosphorylated form in the peripheral blood of PD patients compared with controls are summarized in [Table/Fig-6].
Level of alpha synuclein in the peripheral blood of PD patients from global regions
| Country | Sample Type | Detection method | Sample size (PD/Control) | Average Age in Years (PD/Control); Mean (SD/Range) | Form of Alpha-synuclein | Level of Alpha-synuclein* | Association with PD | Reference |
|---|
| UK | Plasma | ELISA | 34/27 | 65.4 (54–82) /68.0 (55–85) | Oligomeric in Plasma | Higher | Yes | El-Agnaf et al., [6] |
| Spain | Plasma | ELISA | t=42, nt=53/60 | t=65.36 ±1.387, nt=64.26±1.594 / 48.02±2.377 | Total in Plasma | Higher | Yes | Duran et al., [5] |
| Korea | Plasma | ELISA | 105/51 | 64.5±11.4 / 62.8±10.5 | Total in Plasma | Higher | Yes | Lee et al., [1] |
| Australia | Plasma | Western Blotting | 27/11 | 67.2±8.3 / 62.2±9.6 | Total in Plasma | Lower | Yes | Li et al., [7] |
| Germany | CSF | ELISA | 273/23 | 72± 6.8 / 73 ± 8.5 | Total in CSF | Lower | Yes | Mollenhaur et al., [16] |
| Korea | CSF Plasma | ELISA | 23/29 | 62.4±12.7 / 60.1±16.2 | Oligomeric in CSF Total in CSF Oligomeric in Plasma Total in Plasma | Higher No difference No difference No difference | Yes No No No | Park et al., [18] |
| UK | Plasma | ELISA | 32/30 | 68.4 (62.3–738) / 61.5 (42-75) | Phosphorylated in Plasma Total in Plasma Oligomeric in Plasma Oligo-phosphorylated in Plasma | Higher No difference No difference No difference | Yes No No No | Foulds et al., [19] |
| Japan | CSF | ELISA | 32/28 | 67.3 ±9.4 / 64.0 ±13.9 | Total in CSF Oligomeric in CSF | Lower Higher | Yes Yes | Tokuda et al., [17] |
| USA | Plasma | Luminex Assay | 126/122 | 63.7±10.6 / 58.9±17.7 | Total in Plasma | No difference | No | Shi et al., [20] |
| Italy | Plasma | ELISA | 69/110 | 64.59 ± 9.26 / 64.31 ± 9.17 | Total in Plasma | No difference | No | Caranci et al., [9] |
| India | Serum | ELISA | 97/97 | 56.34±9.58/ 53.30±8.33 | Total in Serum | No difference | No | Present Study |
PD= Parkinson’s disease, CSF= cerebro-spinal fluid, t= treated, nt=non-treated
* level of alpha-synuclein in Parkinson’s disease patients when compared with controls
Discussion
The present study was performed in 97 patients with PD and 97 controls. The non-significant differences in age and weight have shown that both the groups were comparable in order to evaluate the levels of total alpha-synuclein and NF-κB in serum from PD patients and controls.
As discussed earlier, the data accumulated for alpha-synuclein in extracellular fluids have been inconsistent, showing evidence for a lower or higher level of total alpha-synuclein in PD patients when compared with controls [1,5,7,15–17]. In the present investigation, the observations indicate that the level of total alpha-synuclein between PD patients and controls were consistent with some earlier studies [8,18–20]. However, these studies were performed in plasma from PD patients. [Table/Fig-6] showed the level of alpha-synuclein in the peripheral blood of PD patients from various countries, but the overall result was inconclusive.
Non-significant difference in the alpha-synuclein level was also supported by the finding of Tan et al., which reported that peripheral alpha-synuclein mRNA expression in sporadic PD did not differ from healthy controls. Hence, significant differential alpha-synuclein mRNA expression did not appear to be a major factor associated with sporadic PD. Because quantitative gene dosage studies in those with highest mRNA expression did not differ from those with the lowest mRNA expression, further suggested that alterations of the gene dose of alpha-synuclein expression levels by duplications or multiplications are not a major cause in the pathogenesis of sporadic PD [21]. This may be a possible reason for the non-significant difference in alpha-synuclein level in the population of the present study.
The variation in alpha-synuclein results could be attributed to the type of detection methods, reagents and antibodies used incubation period, sample size, inadequate numbers of controls and the population studied. Another major factor could be the form of alpha-synuclein that has been measured in a sample, including total alpha-synuclein, oligomeric, phosphorylated or oligophosphorylated [Table/Fig-6].
Similar to alpha-synuclein, lower level of NF-κB was observed in PD patients when compared with controls, but no significant difference was observed in either of the groups. To the best our knowledge, there is no study which reported the presence of NF-κB in the serum of PD patients. So we are unable to compare our results with other studies. Though, NF-κB is localized to the cytoplasm by its inhibitor IκB [22,23], but a study of the mucotoxic drug effect on the serum level of NF-κB has been performed [24].
It is also important to understand the presence of alpha-synuclein and NF-κB in serum. 99 % of the alpha-synuclein in human blood was found to be present in the peripheral blood cells [25] and as for NF-κB, there was a 8-10 fold difference observed between the activated and total amount of NF-κB in peripheral blood mononuclear cells (PBMCs) [10], which suggested that their presence in serum might be due to the interaction with lysed cells.
Further, their levels were analysed considering gender as a factor. Stratified sex analysis showed a non-significant difference in the levels of total alpha-synuclein and NF-κB in serum between males and females in both the groups and within the groups. Thus, it suggested that gender has no influence on the levels of alpha-synuclein and NF-κB in PD patients and controls. Some earlier studies also did not show any difference in the alpha-synuclein expression between males and females or between patients and healthy controls [21,26,27].
Early PD patients and late PD patients also did not show significant differences in the levels of total alpha-synuclein and NF-κB. The levels of total alpha-synuclein and NF-κB were poorly correlated with the present age (at the time of blood sampling) and age at PD onset of the patients. The relationship between age and alpha-synuclein is also controversial. It has previously been reported that alpha-synuclein levels are mainly influenced by gene dosage rather than age [4]; triplication in SNCA gene approximately doubles the amount of alpha-synuclein protein in brain and blood [28]. In the present study, patients with sporadic PD have been taken, which could be a possible reason behind the poor correlation between alpha-synuclein and age. Poor correlation with alpha-synuclein level and disease duration was also found. However, in blood mononuclear cells, the level of alpha-synuclein protein increased significantly with disease duration [29].
Conclusion
The present study concluded that no significant alterations occur in the level of alpha-synuclein and NF-κB in PD patients, which suggested that they are not valuable biomarkers for Parkinson’s disease, more specifically in Indian population. Beside this, gender has no influence on the levels of alpha-synuclein and NF-κB in PD patients.
PD= Parkinson’s disease, n= Number
Values are expressed as mean±SD
PD= Parkinson’s disease, n= Number
Values are expressed as mean±SD
PD= Parkinson’s disease, n= Number
Values are expressed as mean±SD
PD= Parkinson’s disease, CSF= cerebro-spinal fluid, t= treated, nt=non-treated
* level of alpha-synuclein in Parkinson’s disease patients when compared with controls
[1]. Lee PH, Lee G, Park HJ, Bang OY, Joo IS, Huh K, The plasma alpha-synuclein levels in patients with Parkinson's disease and multiple system atrophyJ Neural Transm 2006 113(10):1435-39. [Google Scholar]
[2]. Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjectsNeurosci Lett 2000 287(1):65-67. [Google Scholar]
[3]. Mash DC, Adi N, Duque L, Pablo J, Kumar M, Ervin FR, Alpha synuclein protein levels are increased in serum from recently abstinent cocaine abusersDrug Alcohol Depend 2008 94(1-3):246-50. [Google Scholar]
[4]. Westerlund M, Belin AC, Anvret A, Håkansson A, Nissbrandt H, Lind C, Cerebellar alpha-synuclein levels are decreased in Parkinson's disease and do not correlate with SNCA polymorphisms associated with disease in a Swedish materialFASEB J 2008 22(10):3509-14. [Google Scholar]
[5]. Duran R, Barrero FJ, Morales B, Luna JD, Ramirez M, Vives F, Plasma alpha-synuclein in patients with Parkinson's disease with and without treatmentMov Disord 2010 25(4):489-93. [Google Scholar]
[6]. El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's diseaseFASEB J 2006 20(3):419-25. [Google Scholar]
[7]. Li QX, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Plasma alpha-synuclein is decreased in subjects with Parkinson's diseaseExp Neurol 2007 204(2):583-88. [Google Scholar]
[8]. Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegenerationExp Neurol 2008 213(2):315-25. [Google Scholar]
[9]. Caranci G, Piscopo P, Rivabene R, Traficante A, Riozzi B, Castellano AE, Gender differences in Parkinson's disease: focus on plasma α-synucleinJ Neural Transm 2013 120(8):1209-15. [Google Scholar]
[10]. Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Peripheral cytokines profile in Parkinson's diseaseBrain Behav Immun 2009 23(1):55-63. [Google Scholar]
[11]. Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson’s diseaseProc Natl Acad Sci U S A 1997 94(14):7531-36. [Google Scholar]
[12]. Mogi M, Kondo T, Mizuno Y, Nagatsu T, p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brainNeurosci Lett 2007 414(1):94-97. [Google Scholar]
[13]. Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 casesJ Neurol Neurosurg Psychiatry 1992 55(3):181-84. [Google Scholar]
[14]. Hoehn MM, Yahr MD, Parkinsonism: onset, progression, and mortality. 1967Neurology 2001 57(10 Suppl 3):S11-26. [Google Scholar]
[15]. Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's diseaseBrain 2010 133(Pt 3):713-26. [Google Scholar]
[16]. Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG, α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort studyLancet Neurol 2011 10(3):230-40. [Google Scholar]
[17]. Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson diseaseNeurology 2010 75(20):1766-72. [Google Scholar]
[18]. Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW, Elevated levels of α-synuclein oligomer in the cerebrospinal fluid of drug-naïve patients with Parkinson's diseaseJ Clin Neurol 2011 7(4):215-22. [Google Scholar]
[19]. Foulds PG, Mitchell JD, Parker A, Turner R, Green G, Diggle P, Phosphorylated α-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson's diseaseFASEB J 2011 25(12):4127-37. [Google Scholar]
[20]. Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson's diseaseNeurosci Lett 2010 480(1):78-82. [Google Scholar]
[21]. Tan EK, Chandran VR, Fook-Chong S, Shen H, Yew K, Teoh ML, Alpha-synuclein mRNA expression in sporadic Parkinson's diseaseMov Disord 2005 20(5):620-23. [Google Scholar]
[22]. Scott ML, Fujita T, Liou HC, Nolan GP, Baltimore D, The p65 subunit of NF-kappa B regulates I kappa B by two distinct mechanismsGenes Dev 1993 7(7A):1266-76. [Google Scholar]
[23]. Hayden MS, West AP, Ghosh S, NF-kappa B and the immune responseOncogene 2006 25(51):6758-80. [Google Scholar]
[24]. Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM, Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugsCancer Biol Ther 2008 7(7):1139-45. [Google Scholar]
[25]. Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Red blood cells are the major source of alpha-synuclein in bloodNeurodegener Dis 2008 5(2):55-59. [Google Scholar]
[26]. Michell AW, Luheshi LM, Barker RA, Skin and platelet alpha-synuclein as peripheral biomarkers of Parkinson's diseaseNeurosci Lett 2005 381(3):294-98. [Google Scholar]
[27]. Brighina L, Prigione A, Begni B, Galbussera A, Andreoni S, Piolti R, Lymphomonocyte alpha-synuclein levels in aging and in Parkinson diseaseNeurobiol Aging 2010 31(5):884-85. [Google Scholar]
[28]. Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplicationNeurology 2004 62(10):1835-38. [Google Scholar]
[29]. Fuchs J, Tichopad A, Golub Y, Munz M, Schweitzer KJ, Wolf B, Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brainFASEB J 2008 22(5):1327-34. [Google Scholar]