Introduction
Pain is a sensory perception which is defined by the International Association for the Study of Pain (IASP) for clinical and scientific purposes as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage” [1]. Neuropathic pain originates from damage to central or the peripheral nerves which is due to nerve compression, diabetes, post-stroke, trauma, malignancy, chemotherapy, autoimmune disease and multiple sclerosis etc. [2]. Cancer chemotherapy induced neuropathic pain is the common neurological complication and the onset of symptoms leads to drug discontinuation or dose reduction which can increase cancer related mortality and morbidity [3]. It is estimated that 30–40% of patients receiving chemotherapy eventually develop neuropathic sensory and motor disturbances [4]. Paclitaxel (Taxol) is derived from Pacific yew tree Taxusbrefolia which is used to treat a variety of cancers like ovarian, breast and non-small cell lung cancers. Its effectiveness is limited by the development of severe painful sensory neuropathy thereby reducing the quality of life. The sensory symptoms include paresthesia and dysthesia, pain, numbness, tingling and increased sensitivity to touch and temperature [3,5]. Continuous and multiple dosing may increase the severity of symptoms with loss of deep tendon reflexes, vibratory and sensations. Different group of drugs like tricyclic anti-depressants, anti-epileptics, opioids etc., had been studied in alleviating neuropathic pain induced by chemotherapy and their efficacy is uncertain [6].
With the improved understanding of pathophysiology of neuropathic pain and development of animal models, the efficacy of different groups of drugs are being assessed now a days. Among the anti-epileptics tested carbamazepine and gabapentin have shown promising results in chemotherapy induced neuropathic pain in animal models. Experimental studies showed that gabapentin possess anti-nociceptive effect by reversing allodynia and hyperalgesia due to its action on α2δ1 subunit of voltage sensitive calcium channels thereby inhibiting spontaneous discharge from hyper excited neurones [7]. Pregabalin a newer congener of gabapentin is a selective, high affinity ligand for the α2δ1 protein subunit of voltage-dependent calcium subunit and postulated to modulate the release of several excitatory neurotransmitters thereby interfering with nociceptive signal transfer resulting in analgesic effects on neuropathic pain [8-10].
Many electrophysiological and behavioural studies have used the clinically relevant animal models for evaluating the outcome measures (symptoms associated with neuropathy) in chemotherapy induced neuropathic symptoms [11]. Among the behavioural tests used thermal hypo or hyperalgesia is commonly used to assess the sensory perception disturbances in neuropathic pain. Paclitaxel administration in low cumulative doses as well as high doses induces neuropathic pain manifested as thermal hyperalgesia, cold and mechanical allodynia in rodent models which replicate the clinical symptoms observed in humans [12-14]. Based on the previous studies our study also utilized the standard animal models for testing the neuropathic pain. With this background the present study was designed to study the effect of gabapentin and pregabalin in paclitaxel induced neuropathy in albino wistar rats. We also compared the effect of these drugs in neuropathic pain models.
Materials and Methods
Drugs and Chemicals
Paclitaxel, Gabapentin and Pregabalin were purchased from Clear Synth Lab Pvt. Ltd. India. Hundred percent Acetone was obtained from Sigma Lab Pvt Ltd. The freshly prepared solution of paclitaxel was made in 99.9% HPLC grade methanol (MERCK, USA) obtained from Central Research Laboratory of our research Institute. Fresh solution of Gabapentin and Pregabalin was prepared with 0.9% normal saline prior to each experiment and was given in a dose of 60 and 30 mg/kg body weight respectively. Normal saline (0.9%) was used for vehicle treated animals. The dose selection was done based on previous studies [8,15].
Experimental Animals
Adult male albino wistar rats each weighing 180-250g were used in our study. The animals were acclimatised for a period of seven days in the Department of Pharmacology of our Institute. They were housed in polypropylene cages in a group of six and were maintained under controlled room temperature (25±20C) in a 12 hour light/dark cycle. Animals were fed with standard pellet diet and water ad libitum. The experiment was conducted throughout during the light period especially between 10.00 and 12.00 hours. The study protocol was approved by Institutional animal ethical committee (SMVMCH/ IAEC/DIR/ O.NO13/17/10/12). The care and maintenance of the animals was followed as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines.
Experimental procedure
Rats were divided into four groups with six animals in each group. The animals which exhibited positive withdrawal reaction to painful stimuli was selected and used for this study and then they were randomly divided into four groups as follows: Group 1 received vehicle (0.9 % normal saline), Group 2 received Paclitaxel 2mg/kg (intra peritoneal),Group 3 received Paclitaxel 2mg/kg (intraperitoneal) and Gabapentin 60mg/kg (per-oral) and Group 4 received Paclitaxel 2mg/kg (intraperitoneal) and Pregabalin 30mg/kg (per-oral). Initially behavioural tests were performed for all the four groups and the baseline values were recorded before administering the drugs. Paclitaxel was dissolved in 0.9% saline and was injected in a dose of 2mg/kg intra peritoneally for four alternate days from Day 0 to Day 6 for all the three groups (paclitaxel group and other two drugs treated). For Group 3 and Group 4, Gabapentin and Pregabalin was administered respectively once a day orally from day 0 to day 7 (for 8 consecutive days) along with paclitaxel. Paclitaxel induced neuropathic pain was observed on seventh day which was increasing in intensity on following weeks of observation. The behavioural studies were performed with thermal hyperalgesia and cold allodynia using Radiant heat method and Acetone drop method, Tail Immersion test respectively on day 7, 14, 21 and 28 [16].
Behavioural studies
All behavioural tests were performed by the same person and the observer was blinded during the experimental procedure.
I. Radiant Heat Method
Heat analgesiometer was used for testing Hyperalgesia (Techno, India). It provided pain stimulus by heated nichrome wire with 250v, 50Hz, power 1HP and current was kept constant at 5 ampere for Rat's tail to determine analgesic effect of drugs. The heat source was adjusted to produce a baseline tail flick latency of 3 to 5 seconds (s). A cut-off time of 20 seconds was set to avoid tissue damage. The testing animal was allowed to habituate for approximately 20 minutes or until the exploratory behaviour ceased. Then animal was placed in the restrainer with tail exposed outside. A point between the upper 2/3rd and the lower 1/3rd of the tail was allowed to touch the heat radiant source. The time taken to withdraw the tail from the heat radiant source was noted as the tail withdrawal latency period [16].
II a. Tail Immersion test
Pain elicited by cold is the major feature of many neuropathic pain states. Especially in cold allodynia normal cool stimuli elicits pain in animal models. For testing cold allodynia two standard animal models, namely Acetone drop test and Tail immersion test were used in the present study. In testing cold allodynia using tail immersion test, the terminal part of the tail (5cm distal part) was immersed in a container filled with cold water with 10ºc temperature. Constant temperature (10ºC) was maintained throughout the experiment. Duration of time taken for withdrawal of tail from cold water was noted. A cut-off time of 20 sec was maintained to prevent tissue injury. This procedure was repeated three times for each animal and the mean was calculated. The decrease in tail contact time with cold water was indicative pain whereas prolonged contact time was noted as anti-allodynic effect [16].
II b. Acetone drop method
Cold chemical thermal sensitivity was assessed using acetone drop method as described by Choi et al., with modification [17]. Rats were placed in a metal mesh cage and allowed to habituate for approximately 20 minutes in order to acclimatise them for the new environment. Freshly dispensed acetone drop (50μL) was applied gently on to the mid plantar surface of the hind paw. Cold chemical sensitive reaction with respect to either paw licking, shaking or rubbing the hind paw and brisk foot withdrawal (typically 2–5sec after application) was recorded as a positive response (nociceptive pain response) were as absence or delay of these responses were considered as anti-nociceptive effect. The responses were measured for one minute with a digital stopwatch. For each measurement, the paw was sampled three times for both paws and the mean was calculated. The interval between each application of acetone was approximately 5 minutes.
Statistical Analysis
Data were entered in Epi _info software version 7.0. and analysed using one-way analysis of variance (ANOVA), values were expressed as the Mean ± SD, significance of difference between groups was further analysed by ‘Dunnett’s (2-sided) test for multiple post-hoc comparisons and Mann-Whitney u test. A p-value of < 0.05 was considered statistically significant.
Results
1. The tail withdrawal latency in Radiant Heat Method
Paclitaxel administration resulted a significant (p<0.001) development of thermal hyperalgesia as evidenced by decrease in tail withdrawal latency when compared to vehicle treated group. The intensity of hyperalgesia was maximum at fourth week compared to vehicle treated group [Table/Fig-1,2]. Oral administration of gabapentin (60mg/kg) significantly attenuated (p=0.037 & 0.010) paclitaxel induced thermal hyperalgesia in radiant heat method. The increase in mean tail withdrawl latency was maximum in third and fourth week p= 0.004) than compared to initial two weeks of observation. Oral administration of pregabalin (30mg/kg) also produced a significant inhibition of hyperalgesia (p < 0.001) induced by paclitaxel treatment as evidenced by increase in tail withdrawal latency. This effect was statistically highly significant when compared to gabapentin treated group (p< 0.001).
2. Effect of Gabapentin and pregabalin in cold allodynia
a) Tail Immersion test
In tail immersion method, the tail flicking response was considered as positive response. Gabapentin administration significantly increased the tail flicking response duration from first week to fourth week compared to vehicle (p < 0.001) and paclitaxel treated group (p = 0.003,0.004,0.004 & 0.004). The pregabalin treated group also showed a highly significant prolongation in tail flicking response in all the period of observation compared to vehicle and gabapentin group (p < 0.001) [Table/Fig-3&4].
b). Acetone drop method
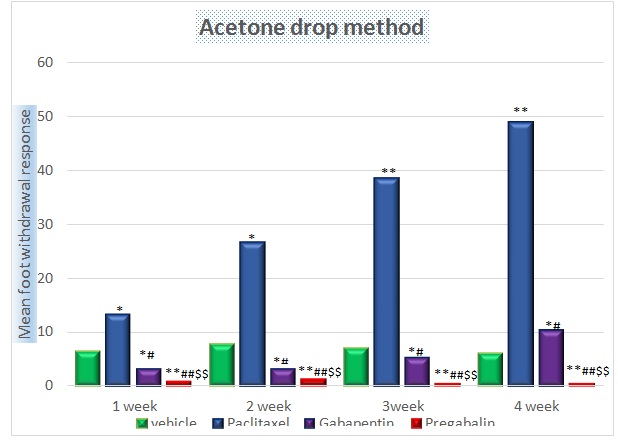
In this method the brisk foot withdrawal response, paw licking, shaking or rubbing the hind paw and its frequency was considered as positive response. Gabapentin treated group resulted a significant reduction in foot withdrawal response when compared to paclitaxel group (p= 0.004 & 0.003). Furthermore in gabapentin group, the foot withdrawal response re-appeared at fourth week. Pregabalin treated group resulted a complete inhibition of foot withdrawal response, paw licking, shaking or rubbing the hind paw noticed in all periods of observation (p< 0.001) when compared to vehicle, paclitaxel and gabapentin treated group [Table/Fig-5].
Discussion
Chemotherapy induced neuropathic pain is common with vincristine, cisplatin, oxaliplatin and paclitaxel therapy [3]. With paclitaxel therapy around 58% of patients develop sensory neuropathy manifested as numbness, tingling and burning pain. Different groups of drugs like opioids, TCA and anticonvulsants have been used in order to prevent or overcome the neurotoxic effects of these compounds. Presently significant improvement of neuropathic pain was observed with the newer antiepileptic agents because of their neuromodulator effects on the hyper excitable damaged nervous system [10].
In the present study, gabapentin treated group significantly attenuated paclitaxel induced hyperalgesia as evidenced by increased tail withdrawal latency using radiant heat method for initial two weeks of observation when compared with vehicle and paclitaxel treated group. Furthermore, increase in tail withdrawal latency was also observed in third and fourth weeks of observation as compared to vehicle and paclitaxel treated animals. This was supported by Paudel KR et al., who demonstrated the significant anti-nociceptive effect of gabapentin given alone than compared to other anti-epileptics like lamotrigine and topiramate [18]. In addition, combination of gabapentin with lamotrigine and topiramate showed significant anti-nociceptive effect than monotherapy was also shown by the same study [18]. However, in our study effect of combination of drugs was not studied. Pregabalin treated group also showed significant increase in tail withdrawal latency when compared with paclitaxel group in all the four weeks of observation. Moreover, the pregabalin treated group significantly inhibited thermal hyperalgesia when compared with gabapentin treated animals during the study period. This result was in concordance with similar study conducted by Hue jung park and co-workers as dose related effect of pregabalin in peripheral neuropathy [8]. Pregabalin administered by different routes (Intrathecal and intraperitoneal routes) produced significant anti-nociceptive effect was also shown in yet another study by Kumar N et al., [9].
Both gabapentin and pregabalin group showed anti-allodynic effect in all four weeks of observation in tail immersion test. Earlier study conducted by Vinay kumar and his co-workers also showed similar significant anti-allodynic effect with gabapentin treatment than compared to lamotrigine treatment [19]. Our study showed sustained antiallodynia with pregabalin treatment than that of gabapentin in all the four weeks of observation. The anti-allodynic effect of pregabalin was maximum for a period of 12 days in yet another study, whereas in our study, pregabalin had produced prolonged effect (4 weeks) [9]. This was further confirmed by absence of foot withdrawal, paw licking, shaking or rubbing the hind paw during the observation time of one minute in all the four weeks of the study. Gabapentin produced significant effect in initial two weeks but this effect was reduced on fourth week indicating its mild decrease in anti-allodynic effect by acetone drop test. One study conducted on neuropathy pain induced by oxaliplatin stated that intrathecal administration of pregabalin produced antiallodynia but our study showed better anti-allodynic effect with oral administration of pregabalin in acetone drop method [20]. Though reports are available indicating that gabapentin and pregabalin have significant anti-nociceptive effects against nociceptive and inflammatory pain their efficacy for attenuating paclitaxel-induced neuropathic pain was not established. In the present study, repeated administration of gabapentin and pregabalin significantly attenuated paclitaxel induced neuropathic pain during all four weeks of observation.
The precise mechanism of sensitivity to heat and cold exposure observed with paclitaxel was not clearly understood and it was postulated that paclitaxel probably causes dysfunctional microtubules in dorsal root ganglia, axons and schwann cells [21]. Previous experimental studies had shown that gabapentin binds with a high affinity to α2δ1-subunit of voltage dependent calcium channels and reduces glutamate release by reducing pre-synaptic calcium influx [9]. This possible mechanism could account for the anti-nociceptive effect of gabapentin [9]. However, pregabalin binds high affinity to α2δ1-subunit of voltage- gated calcium (Ca2+) channels and attenuates the neuronal calcium influx thereby causing inhibition of the release of neurotransmitters including norepinephrine, substance P and glutamate [9]. This mechanism may be responsible for the highly significant attenuation of pain induced by paclitaxel in all the three animal models.
The above findings suggested that systemic administration of gabapentin and pregabalin has significant anti-hyperalgesic and anti-allodynic effect. In addition pregabalin reported to have significant anti-hyperalgesia and anti-allodynic effect than compared to gabapentin which could be due to its selective, high affinity towards α2δ1 protein subunit of voltage dependent Ca2+ channels. The study limitations are our test procedure employed only single dose level, short duration of follow up and involving only three animal models. However further studies using different doses involving other nociceptive models may add authenticity to the present results.
The tail withdrawal latency of rats treated with gabapentin and pregabalin by radiant heat method (initial two weeks of treatment)
| Groups | Treatment (mg/kg i.p/oral) | Withdrawal Latency (Seconds) |
|---|
| 1st week | p-value | 2nd week | p-value |
|---|
| Group 1 | Vehicle | 7.66 ± 1.03 | | 8 ± 1.41 | |
| Group 2 | Paclitaxel (2) | * 4.16 ± 1.17 | 0.01 | * 4.16 ± 0.75 | 0.01 |
| Group 3 | Gabapentin (60) + Paclitaxel (2) | * # 9.55 ± 1.38 | 0.037 | * # 11.66 ± 1.86 | 0.010 |
| Group 4 | Pregabalin (30) + Paclitaxel (2) | ** ## $$ 12.16 ± 1.47 | <0.001 | ** ## $$ 14.33 ± 2.5 | < 0.001 |
Values are expressed as Mean ± SD for all four groups (n = 6)
*p < 0.01, **p < 0.001 compared to vehicle group
#p < 0.01,##p < 0.001 compared to paclitaxel group
$p < 0.01, $$p < 0.001 pregabalin compared with gabapentin
Comparison was done by one-way ANOVA followed by Post-hocDunnett’s (2-sided) test and Mann Whitney U-test
The tail withdrawal latency of rats treated with gabapentin and pregabalin by radiant heat method (third and fourth weeks of treatment)
| Groups | Treatment (mg/kg i.p/oral) | Withdrawal Latency (Seconds) |
|---|
| 3rd week | p-value | 4th week | p-value |
|---|
| Group 1 | Vehicle | 8.16 ± 1.60 | | 8 ± 1.09 | |
| Group 2 | Paclitaxel (2) | ** 3.16 ± 1.17 | < 0.001 | ** 2.33 ± 0.52 | < 0.001 |
| Group 3 | Gabapentin (60) + Paclitaxel (2) | ** ## 14.16 ± 1.47 | 0.004 | ** ## 14.66 ± 1.97 | 0.004 |
| Group 4 | Pregabalin (30) + Paclitaxel (2) | ** ## $$ 17.5 ± 0.84 | < 0.001 | ** ## $$ 18.66 ± 1.21 | < 0.001 |
Values are expressed as Mean ± SD for all four groups (n = 6)
*p < 0.01, ** p < 0.001 compared to vehicle group
#p < 0.01, ## p < 0.001 compared to paclitaxel group
$p < 0.01, $$ p < 0.001 pregabalin compared with gabapentin
Comparison was done by one-way ANOVA followed by Post-hoc Dunnett’s (2-sided) test and Mann-Whitney U-test
The tail flicking response of rats treated with gabapentin and pregabalin by tail immersion test (initial two weeks of treatment)
| Groups | Treatment (mg/kg i.p/oral) | Withdrawal Latency (Seconds) |
|---|
| 1st week | p-value | 2nd week | p-value |
|---|
| Group 1 | Vehicle | 8.33 ± 1.16 | | 8.66 ± 0.82 | |
| Group 2 | Paclitaxel (2) | * 6.83 ± 0.75 | 0.006 | * 5.66 ± 0.82 | < 0.001 |
| Group 3 | Gabapentin (60) + Paclitaxel (2) | ** ## 13.66 ± 0.82 | 0.003 | ** ## 13.6 ± 2.07 | 0.004 |
| Group 4 | Pregabalin (30) + Paclitaxel (2) | ** ## $$ 18.66 ± 1.21 | < 0.001 | ** ## $$ 19 ± 0.89 | < 0.001 |
Values are expressed as Mean ± SD for all four groups (n = 6)
*p < 0.01, **p <0.001 compared to vehicle group
#p < 0.01#,##p <0.001 compared to paclitaxel group
$p < 0.01, $$p <0.001 pregabalin compared with gabapentin
Comparison was done by one-way ANOVA followed by Post-hocDunnett’s (2-sided) test and Mann-Whitney U-test
The tail flicking response of rats treated with gabapentin and pregabalin by tail immersion test (third and fourth weeks of treatment)
| Groups | Treatment (mg/kg i.p/oral) | Withdrawal Latency (Seconds) |
|---|
| 3rd week | p-value | 4th week | p-value |
|---|
| Group 1 | Vehicle | 8.16 ± 1.17 | | 7.83 ± 0.75 | |
| Group 2 | Paclitaxel (2) | ** 3.67 ± 1.17 | < 0.001 | ** 2.33 ±0.52 | < 0.001 |
| Group 3 | Gabapentin (60) + Paclitaxel (2) | ** ## 16.66 ± 0.82 | 0.004 | ** ## 16.6 ± 1.21 | 0.004 |
| Group 4 | Pregabalin (30) + Paclitaxel (2) | ** ## $$ 19.33 ± 0.81 | < 0.001 | ** ## $$ 19.5 ± 0.84 | < 0.001 |
Values are expressed as Mean ± SD for all four groups (n =6)
*p < 0.01, **p < 0.001 compared to vehicle group
#p < 0.01#, ## p < 0.001 compared to paclitaxel group
$p < 0.01, $$p < 0.001 pregabalin compared with gabapentin
Comparison was done by one-way ANOVA followed by Post-hoc Dunnett’s (2-sided) test and Mann-Whitney U-test
The Brisk Foot Withdrawal Response of rats treated with Gabapentin and Pregabalin by Acetone drop method
Values are expressed as Mean ± SD for all four groups (n = 6)
*p < 0.01, ** p < 0.001 compared to vehicle group
#p < 0.01,##p < 0.001 compared to paclitaxel group
$p < 0.01, $$p < 0.001 pregabalin compared with gabapentin
Comparison was done by one-way ANOVA followed by Pos-hoc Dunnett’s (2-sided) test and Mann Whitney U-test
Conclusion
From this study it was concluded that gabapentin and pregabalin treated animals decreased thermal hyperalgesia and possessed anti-allodynic effect. Pregabalin was most effective in attenuating paclitaxel induced neuropathic pain when compared to gabapentin. More detailed studies in various doses using different animal models followed by human trials may help to identify the effect of these drugs in cancer chemotherapy induced neuropathy pain.
Acknowledgement
We would like to thank our institution for permitting and providing us the necessary assistance for the study.
Values are expressed as Mean ± SD for all four groups (n = 6)*p < 0.01, **p < 0.001 compared to vehicle group#p < 0.01,##p < 0.001 compared to paclitaxel group$p < 0.01, $$p < 0.001 pregabalin compared with gabapentinComparison was done by one-way ANOVA followed by Post-hocDunnett’s (2-sided) test and Mann Whitney U-test
Values are expressed as Mean ± SD for all four groups (n = 6)*p < 0.01, ** p < 0.001 compared to vehicle group#p < 0.01, ## p < 0.001 compared to paclitaxel group$p < 0.01, $$ p < 0.001 pregabalin compared with gabapentinComparison was done by one-way ANOVA followed by Post-hoc Dunnett’s (2-sided) test and Mann-Whitney U-test
Values are expressed as Mean ± SD for all four groups (n = 6)*p < 0.01, **p <0.001 compared to vehicle group#p < 0.01#,##p <0.001 compared to paclitaxel group$p < 0.01, $$p <0.001 pregabalin compared with gabapentinComparison was done by one-way ANOVA followed by Post-hocDunnett’s (2-sided) test and Mann-Whitney U-test
Values are expressed as Mean ± SD for all four groups (n =6)*p < 0.01, **p < 0.001 compared to vehicle group#p < 0.01#, ## p < 0.001 compared to paclitaxel group$p < 0.01, $$p < 0.001 pregabalin compared with gabapentinComparison was done by one-way ANOVA followed by Post-hoc Dunnett’s (2-sided) test and Mann-Whitney U-test
[1]. HBN Merskey, Part III: Pain terms, a current list with definitions and notes on usageIASP Press 1994 6:249-52. [Google Scholar]
[2]. M Haanpaa, RD Treede, Diagnosis and classification of neuropathic painPain: Clinical Updates 2010 18:1-6. [Google Scholar]
[3]. J Ferrier, V Pereira, J Busserolles, N Authier, D Balayssac, Emerging trends in understanding chemotherapy-induced peripheral neuropathy.Current Pain and Headache Reports 2013 17(10):364-71. [Google Scholar]
[4]. Guo Zheng-gang, Jia Xiao-pen, Su Xiao-jun, Li Ping, Hao Jian-hua, Gastrodin attenuates vincristine-induced mechanical hyperalgesia through serotonin 5-HT1A receptorsBangladesh J Pharmacol 2013 8:414-19. [Google Scholar]
[5]. C Visovsky, M Collins, L Abbott, J Aschenbrenner, C Hart, Putting evidence into practice: Evidence-based interventions for chemotherapy-induced peripheral neuropathyClin J OncolNurs 2007 11:901-13. [Google Scholar]
[6]. RD Rao, JC Michalak, JA Sloan, CL Loprinzi, GS Soori, DA Nikcevich, Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized double-blind, placebo-controlled, crossover trial (N00C3)Cancer 2007 110(9):2110-18. [Google Scholar]
[7]. HL Pan, JC Eisenach, SR Chen, Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic ratsJ Pharmacol ExpTher 1999 288:1026-30. [Google Scholar]
[8]. HJ Park, HS Joo, HW Chang, JY Lee, SH Hong, Y Lee, Attenuation of neuropathy-induced allodynia following intraplantar injection of pregabalinCan J Anaesth 2010 57(7):664-71. [Google Scholar]
[9]. N Kumar, A Laferriere, JS Yu, A Leavitt, TJ Coderre, Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamateJournal of neurochemistry 2010 113:552-61. [Google Scholar]
[10]. JO McNamara, Pharmacotherapy of the Epilepsies. In: Brunton LL, Chabner BA, Knollman BC. Goodman & Gilman’s The Pharmacological Basis of Therapeutics 2011 12th EditionNew YorkMcGraw Hill:583-607. [Google Scholar]
[11]. N Authier, D Balayssac, F Marchand, B Ling, A Zangarelli, J Descoeur, Animal Models of Chemotherapy-Evoked Painful Peripheral NeuropathiesNeurotherapeutics 2009 6(4):620-29. [Google Scholar]
[12]. N Authier, JP Gillet, J Fialip, A Eschalier, F Coudore, Description of a short-term Taxol-induced nociceptive neuropathy in ratsBrain Res 2000 887(2):239-49. [Google Scholar]
[13]. JP Cata, HR Weng, PM Dougherty, The effects of thalidomide and minocycline on taxol-induced hyperalgesia in ratsBrain Res 2008 1229:100-10. [Google Scholar]
[14]. AS Jaggi, V Jain, N Singh, Animal models of neuropathic painFundamental & Clinical Pharmacology 2011 25:1-28. [Google Scholar]
[15]. B Chogtu, KL Bairy, D Smitha, S Dhar, P Himabindu, Comparison of the efficacy of carbamazepine, gabapentin and lamotrigine for neuropathic pain in ratsIndian J Pharmacol 2011 43(5):596-98. [Google Scholar]
[16]. L Saha, S Hota, A Chakrabarti, Evaluation of lercanidipine in Paclitaxelinduced neuropathic pain model in rat: a preliminary studyPain Res Treat 2012 2012(1):143579 [Google Scholar]
[17]. Y Choi, YW Yoon, HS Na, SH Kim, JM Chung, Behaviouralsigns of ongoing pain, cold allodynia in a rat model of neuropathic painPain 1994 59(3):369-76. [Google Scholar]
[18]. KR Paudel, S Bhattacharya, G Rauniar, B Das, Comparison of anti-nociceptive effect of the antiepileptic drug gabapentin to that of various dosage combinations of gabapentin with lamotrigine and topiramate in mice and ratsJ Neurosci Rural Pract 2011 2(2):130-36. [Google Scholar]
[19]. V Kumar, M Ashutosh, K Nagarajan, BK Singh, B Umakant, Ameliorative Effect of Green Lipped Mussel Extract on Vincristine-Induced Painful Neuropathy in RatsJ Pharmacol Drug Metab 2014 1:103 [Google Scholar]
[20]. B Ling, F Coudore, L Decalonne, A Eschalier, N Authier, Comparative anti-allodynic activity of morphine, pregabalin and lidocaine in a ratmodel of neuropathic pain produced by one oxaliplatin injectionNeuropharmacology 2008 55(5):724-28. [Google Scholar]
[21]. V Vuorinen, M Roytta, CS Raine, The acute response of Schwann cells to taxol after nerve crush.Acta Neuropathol (Berl) 1988 76:17-25. [Google Scholar]