In the global tuberculosis report (2014), WHO reported that in 2013, 9 million people developed TB, including 1.1 million cases among people who were HIV positive. At the same time, global burden of multidrug-resistant TB (MDR-TB) was estimated to be 480,000 cases leading to estimated 210,000 deaths [1]. Twenty five percent of global annual TB incidents occur in India making it highest Tuberculosis burden country [2]. One-fourth of TB cases notified are Extrapulmonary and incidence is even higher in children and in immunocompromised people [3]. Prompt and accurate TB diagnosis are prerequisite for early and effective treatment thereby reducing tuberculosis burden, but there are number of difficulties in achieving the goal. Sputum smear microscopy is inefficient due to its variable sensitivity particularly in patients with sputum smear-negative and/or extrapulmonary disease, and drug-resistant TB [4–6]. Latest generation liquid culture diagnostics and molecular line probe assays are costly and cannot be performed in resource-limited settings, due to need for biosafety measures and specialised staff [4]. Besides technical expertise and biosafety concerns, Lowenstein-Jensen (LJ) method, “the gold standard test”, takes several weeks to produce result causing delayed onset of treatment [6]. In December 2010, WHO recommended use of a new Cartridge Based Nucleic Acid Amplification test (CB-NAAT), named GeneXpert system [1]. The Xpert MTB/RIF assay employs five distinct molecular beacons (nucleic acid probes), each labelled with a differentially coloured fluorophore and responding to a specific nucleic acid sequence within the rpoB gene of M. tuberculosis [3,4]. It can detect TB along with rifampicin resistance in less than two hours, directly from untreated sputum samples [3,7].
Revised National TB Control Programme (RNTCP) is also currently using Xpert MTB/RIF to diagnose pulmonary TB, paediatric TB, extrapulmonary TB and rifampicin resistance and Multi Drug Resistance Tuberculosis in high risk populations like HIV positive as recommended by WHO under 2013 policy recommendations [1,3,7].
Materials and Methods
This study is done at GGSM College, Faridkot a leading tertiary care centre of Malwa region of Punjab, India. Sputum samples are received from 5 districts of Punjab (Bathinda, Faridkot, Moga, Kothpura, Ferozpur, India). The study period is from October 2013 to September 2014. A total of 907 sputum samples of the patients with symptoms suggestive of pulmonary tuberculosis including both new cases and on treatment were received from the various district and civil hospitals of 5 districts. All specimens were collected in pre-sterilized falcon tubes with three layer packing system, after through rinsing of the oral cavity with clean water. Samples along with prescribed Performa containing details of patients like Name, Address, Age, Sex, HIV status, and Name of the referring centre was received in the Microbiology Laboratory.
TB detection was done by Xpert MTB/RiF assay, made by Cepheid-Sunnyvale-USA. Sputum specimens were processed according to the GeneXpert Dx system operator manual given by Central TB division, Government of India , Guidance document for use of cartridge based nucleic acid amplification test (CB-NAAT) under RNTCP [7,8]. The assay is designed for extraction, amplification and identification of rpoB gene of M. tuberculosis as it accounts for more than 95% of mutations associated with rifampicin resistance), ensuring high degree of specificity by use of three specific primers and 5 unique molecular probes [9]. The number of positive beacons, their detection timing (indicated by rise of fluorescent signal above a predetermined baseline cycle threshold) and the results of sample processing controls, allow the test to distinguish among the following results: no TB; TB detected, rifampicin resistance detected; TB detected, no rifampicin resistance detected; TB detected, rifampicin resistance indeterminate; and an invalid result [3,4]. Xpert MTB/RIF cartridge is a disposable, single self-enclosed test unit in which all steps of NAAT i.e. Sample processing, PCR amplification and detection are automated and integrated. The manual steps involved in the assay are adding reagent to liquefy sputum and sample loading [10]. The test procedure is made biosafe by tuberculocidal property of the assay’s sample reagent [3].
Results
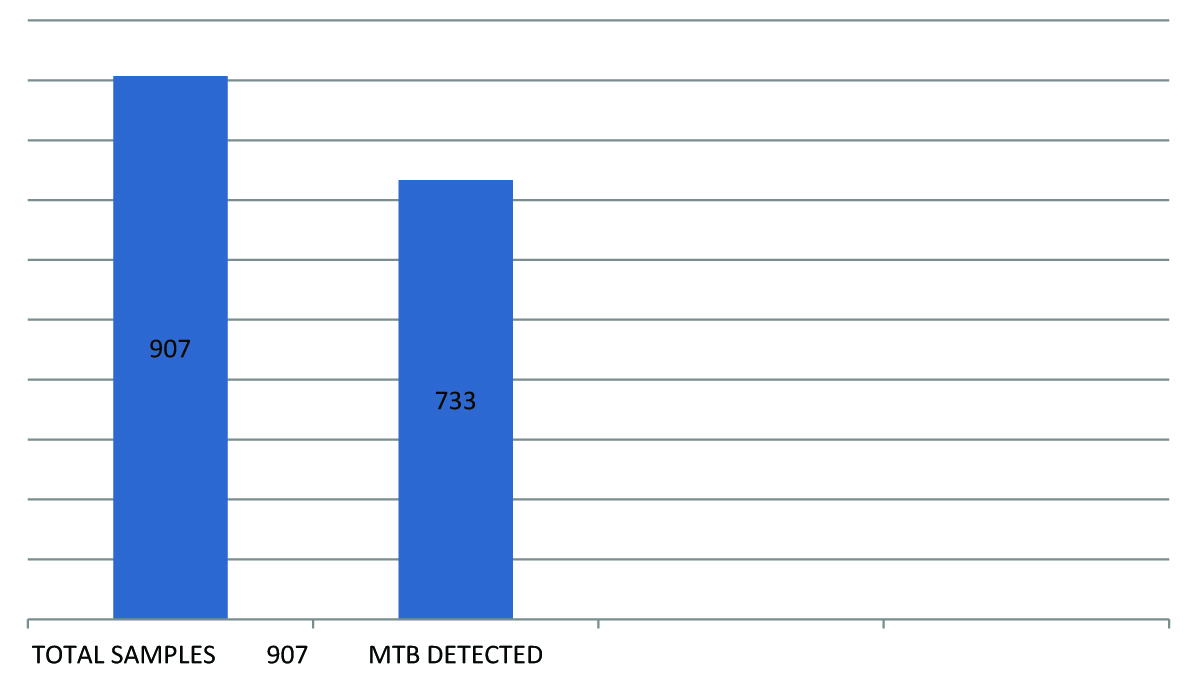
Of total 907 sputum smear confirmed cases, included in study, MTB was detected in 733 patients as shown in [Table/Fig-1].
Total sputam smear confirmed cases included in study and MTB detected cases
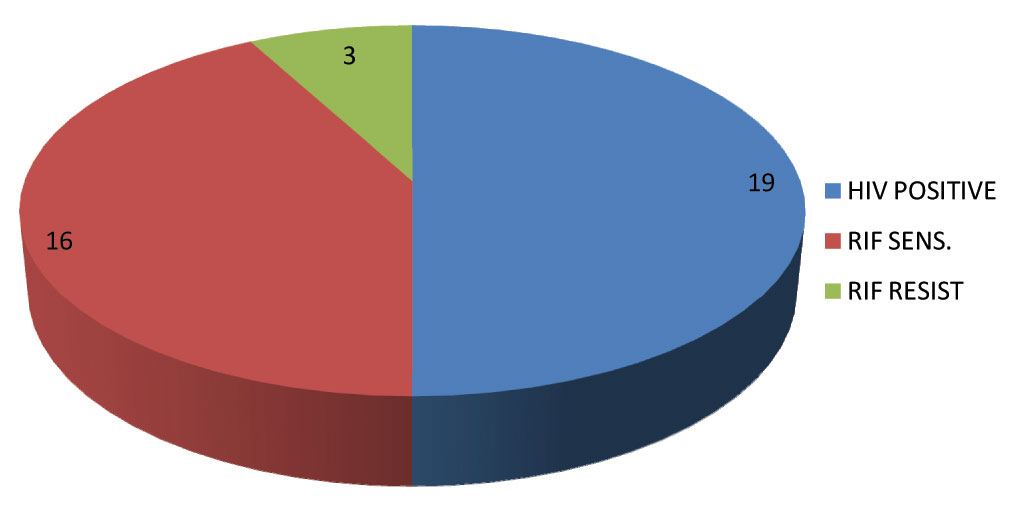
Out of these 733 patients which were reported MTB positive 19(2.5%) were reported positive for (HIV +TUBERCULOSIS). Of these 19 Co-infected cases 16 (84.21%) cases were sensitive to rifampicin (RiF) and 3(15.78%) cases were showing resistance to rifampicin (RiF) Drug as shown in [Table/Fig-2].
TB-HIV Co-infected, cases sensitive to rifampicin (RiF) and cases showing resistance to rifampicin (RiF)
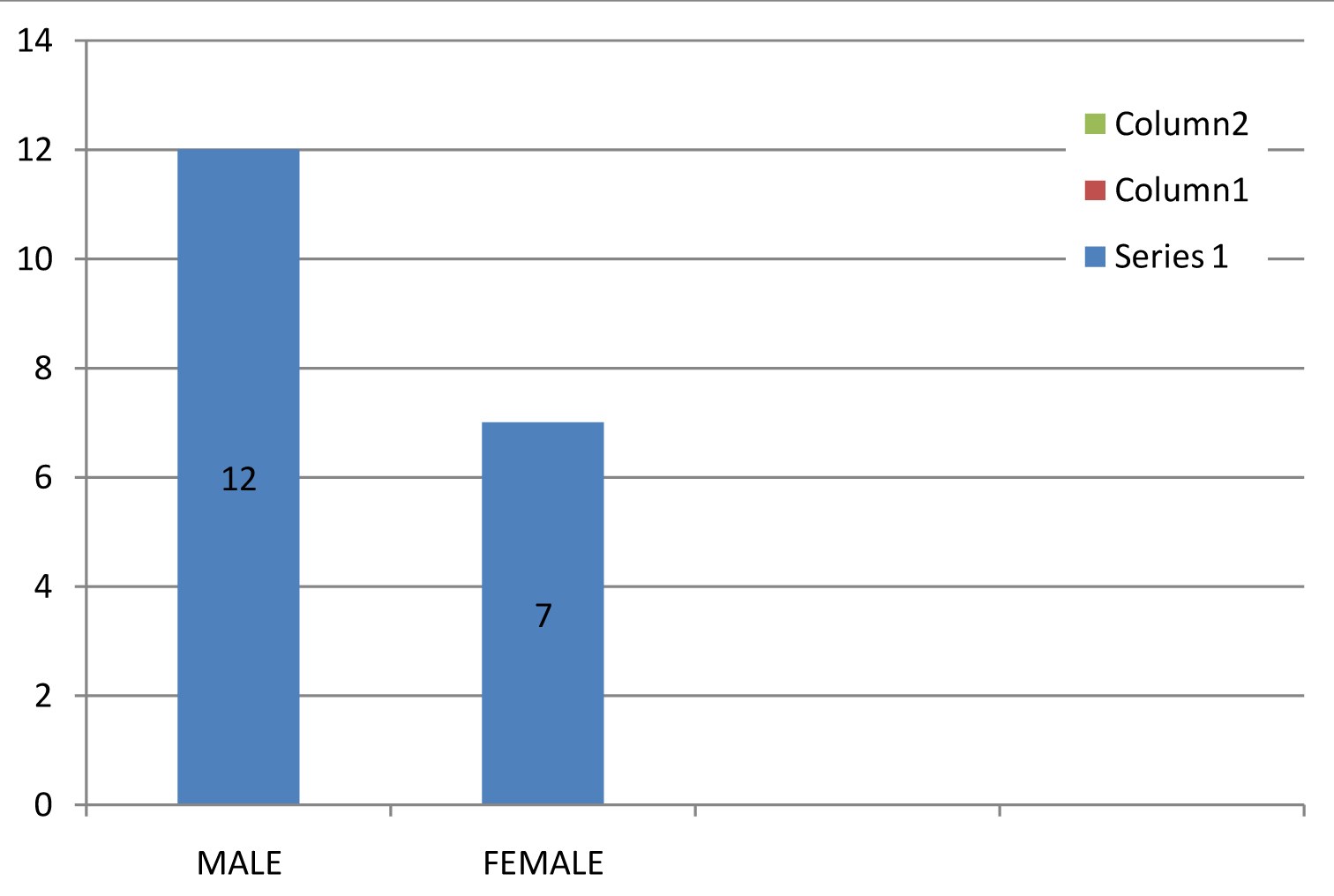
Sex wise distribution showed that 12(63.15%) were male and 7(36.84%) were female as in [Table/Fig-3].
HIV-TB co-infected males and females
Age wise distribution shows of the 19 HIV positive patients all were in the age group of more than 20 y.
Discussion
In 2013, 48% of TB patients globally had a documented HIV test result [1]. India accounts for about 10% of the global burden of HIV-associated TB. The mortality in this group is very high and every year: 42000 people die every year among TB/HIV co-infected patients [2].
In given study 2.6% patients were found to be HIV–TB co-infected, of which 12(63.15%) were males and 7(36.48%) were females. Male predominance has been observed in other studies from Punjab too [11,12]. In a Study conducted in AIIMS-New Delhi for period of six years, HIV-TB co-infection was diagnosed in 33.2% patients of which 81.3% were male [13]. This male predominance may be accounted their migration for employment within and outside the state thereby subjecting them to risk behaviour. Majority of female are illiterate and are house-wives who don’t even have easy access to healthcare facilities, and HIV and TB both being social stigma often go unreported.
As per the WHO Global Report on Tuberculosis 2013, India accounts for 64,000 MDRTB cases out of 300,000 cases estimated globally to occur among the notified pulmonary TB cases annually [2]. High prevalence of MDR-TB was detected among HIV positive cases (15.78%) which in accordance with the study done by Sethi et al., at PGIMER, Chandigarh for a span of 41 months who reported significantly higher association of MDR-TB (27.3%) with HIV seropositive patients as compared to HIV seronegative patients (15.4%) [12].
Cartridge Based Nucleic Acid Amplification testing (CB-NAAT) is a new operational system recommended by WHO. Results from 12 single centre evaluation studies with varying design and study population and reviewed by WHO reported the sensitivity in detecting TB from 70%-100% in culture positive patients and around 60% in those with smear negative disease and specificity ranging from 91%-100% and average rifampicin sensitivity and specificity around 98% and 99% [7].
Studies from different part of world have reported high sensitivity and specificity by using Xpert MTB/RIF test based on this assay. In a study by Raizada et al., that covered a population of 8.8 million across 18 sub-district level tuberculosis units (TU) in India, Overall 28% TB cases were bacteriologically confirmed, of which 27.6% TB cases were detected on Xpert MTB/RIF against smear positivity rate of 12.9%. However, of 9 Xpert MTB/RIF negative and culture positive cases, 8 were detected on smear microscopy too. Positive predictive value (PPV) for rifampicin resistance detection by Xpert MTB/RIF was 97.7% [14]. In a similar study, Raizada et al., reported Among the 485 bacteriologically-confirmed paediatric (0-14 y) PTB cases, 98.4% had a positive TB result on Xpert MTB/RIF and of these 54.9% were Xpert MTB positive and smear negative [15]. Similar study on HIV positive adults referred to DOTS centre with pulmonary symptoms suggestive of tuberculosis Singh and co-authors concluded that “CBNAAT is easily done, valid, more accurate and reliable alternative to sputum microscopy for detection of pulmonary tuberculosis in HIV patients” [16].
Steingart et al., performed an updated Cochrane Review as part of a WHO process to develop updated guidelines on the use Xpert® MTB/RIF assay and reported test to be sensitive and specific In adults thought to have TB, with or without HIV infection,. Compared with smear microscopy, Xpert® MTB/RIF substantially increases TB detection among culture-confirmed cases. For rifampicin resistance detection, Xpert® MTB/RIF provides accurate results and can allow rapid initiation of MDR-TB treatment [17].
Few studies have given contradictory results too as in a study conducted in Durban, South Africa on patients detected with rifampicin resistance on Xpert test, The Xpert MTB/RIF assay showed 8.8% discordance when compared to culture and the overall rate of rifampicin monoresistance was 13.4% [18]. The specificity of Xpert MTB/RIF in detecting rifampicin resistance is very high (98%), and increasing evidence has shown that the infrequent occurrence of so-called false-positive results may be linked to the detection by Xpert MTB/RIF of strains that are truly resistant to rifampicin, but which are not detected by the phenotypic culture-based DST, the present reference standard [9]. A study on previously treated TB patients showed false positivity by Xpert MTB/RIF assays due to presence of dead Mycobacterium tuberculosis bacilli in lungs and sputum [19].
However, cost of Xpert assay is more than smear microscopy; it requires a machine that currently costs US$17,000 and cartridges that cost US$9.98 for each test, in addition to human resource and other running costs [20]. Vassall et al., reported the cost for the Xpert test (including all costs, such as the cartridge, equipment, salaries) ranges from US$22.63 in India to US$27.55 in Uganda [21]. Due to IPAQT (www.ipaqt.org; a coalition of private labs in India, supported by industry and nonprofit groups), the maximum price labs can charge patients for Xpert MTB/RIF is now Rs. 1700 (Indian Rupees) [22].
There are few limitations with device like incomplete sensitivity for smear-negative TB and rifampicin resistance as well inability to detect resistance to isoniazid and other drugs, nonstop power supply, Ambient temperature not exceeding 30°C, Biosafety comparable to smear microscopy, Waste disposal system for cartridges, Trained laboratory and clinical staff, Annual calibration of the Xpert modules, Review (and revision) of diagnostic algorithms, policies, forms, and guidance [21].
The major limitation of the present study is the small sample size and therefore, it is not representative of the population at large. In fact, this limitation was observed in most previous studies of HIV and MDR-TB. Data regarding prevalence of TB and its drug resistance status at national and State level are pressing need to design strategy against them.
Conclusion
In the rapidly evolving era of TB and HIV co-infection, updated knowledge as well as changing research priorities, particularly with respect to new TB diagnostics is the need of the hour. The Gene Xpert system can be used as the initial diagnostic test in individuals suspected of having MDR-TB or HIV associated TB as it can test 4 modules with capacity to perform 15 to 20 tests in one working day and the result is available in less than 2 h and hence screening capacity of healthcare centre can be increased. Use of Xpert has significantly increased TB finding and it has also significantly increased MDR case finding and cost wise it is much less than performing culture and DST. The system can be operated under diverse environmental conditions, with minimally trained staff and least biosafety concerns. But every packaged deal has its own share of negativities and in this, device maintenance, annual calibration of instrument modules is required along with constant electricity supply, which may pose problems in most endemic remote area. Besides, to test MDR and resistance to other drugs, DST is required.