Aims and Objectives
To study the effect of dexamethasone on liver and endothelium, and to determine the optimum dose which induces the abnormal changes in liver and endothelium in Wistar rats.
Materials and Methods
Albino Wistar rats were divided into 7 groups (n=6). Control group rats received normal saline. Graded doses of dexamethasone (0.5,1,2,4,8 and 16mg/kg/ i.p.) was administered to the groups for six days. Liver and aorta were dissected at the end of the study and examined for histopathological changes under microscope.
Results
Intraperitoneal administration of dexamethasone (4,8 and 16mg/kg) for six days resulted in fatty changes in liver and same doses have shown thickening of endothelial layers in aorta, in comparison to control group. There were not much significant changes seen in low doses of dexamethasone (0.5, 1 and 2mg/kg).
Conclusion
It is concluded that the acute high doses of dexamethasone (4,8 and 16mg/kg) for six days caused hepatic steatosis and showed mild to moderate arteriosclerosis in aorta. These changes may be secondary consequences of insulin resistance. Hence, it can be used as new animal model to screen the various plants and medicines in the treatment of insulin resistance.
Introduction
Glucocorticoids (GC) are most commonly used drugs in several clinical conditions such as rheumatoid arthritis, cerebral edema, asthma and as immunosuppressant in certain organ trans- plantation [1]. Up to 2.5% of the population takes prescribed glucocorticoids and their side effects represent a considerable clinical burden [2]. Glucocorticoids (GCs) in general increase blood glucose levels by following mechanisms; increased hepatic glucose production (gluconeogenesis), decreased peripheral glucose uptake into muscle and adipose tissue, breakdown of muscle and fat to provide additional substrates for glucose production and inhibition of insulin release from pancreatic β cells [1,3]. Prolonged GC exposure (as with therapeutic use of GCs or in Cushing syndrome), insulin secretion will increase to compensate for the excess glucose levels and ultimately results in severe insulin resistance and metabolic dysfunction, however the precise molecular mechanism is not clear [1].
Insulin resistance is a condition associated with reduced responsiveness of a target cell or a whole organism to the insulin concentration to which it is exposed, in which the circulating insulin levels are higher than the required quantity leading to several therapeutic problems [4]. Certainly high therapeutic doses of GC was prone for development of insulin resistance associated with type 2 diabetes, cardiovascular problems, hypertension, dyslipidemia and hepatic steatosis [5,6].
The liver is also the primary metabolic target of GC action. Liver is the key organ that contributes to insulin resistance through increasing glucose output. A positive relationship has been proposed between glucocorticoid effects in the liver and whole body insulin resistance [7]. Non alcoholic fatty liver associates with an imbalance of fat metabolism in the liver. It mainly results from excessive fatty acid synthesis or uptake. GCs are known to contribute to fatty liver production through a combination of increased fatty acid synthesis and decreased fatty acid β oxidation. Fatty liver induced by glucocorticoids is mainly of resistance to insulin action [8].
Insulin resistance individuals are at 2.5 times higher risk of dying with cardiovascular problems than non insulin resistance individuals [9]. It occurs due to dyslipidemia, endothelial dysfunction and sympathetic over activity, etc [10]. Glucocorticoids administration causes insulin resistance leading to various cardiovascular complications [7]. Glucocorticoid induces changes in endothelium and also decreases the aortic compliance, which may be secondary to insulin resistance [11].
Dexamethasone is a potent glucocorticoid with minimal mineralocorticoid property; it has high affinity for glucocorticoid receptors. Several studies were conducted for inducing insulin resistance by use of dexamethasone. The major advantage of using dexamethasone is that, it can induce insulin resistance in rodents in a relatively short period of time [12,13]. According to literature survey, dexamethasone can induce insulin resistance. But, there is scarce of data on dexamethasone associated complications on liver and endothelium due to insulin resistance. Hence, we tried to establish a new animal model, to understand the pathological process of insulin resistance and to screen the maximum dose of dexamethasone which can induce changes in histology of liver and endothelium.
Materials and Methods
The present study was conducted on male albino wistar rats of weight 230-300gms. Animals were obtained from central animal house of the institution. They were maintained under standard conditions approved by Committee for the purpose of control and supervision on experimental animals (CPCSEA), temperature of (23±2)0C, humidity 50±5%, 12hr light: dark cycles. Animals were housed in polypropylene cages (UN Shah Manufactures, Mumbai, India), provided with standard food (Hindustan lever ltd, Mumbai, India) and water ad libitium. Institutional animal ethical committee permission was obtained prior to study. Dexamethasone was procured from Zydus pharmaceuticals, Mumbai, India.
Study design
Animals were divided into seven groups. Animals in Group 1 treated with normal saline (i.p.) considered as normal control. The animals in group 2 to 7 were treated with dexamethasone (0.5, 1, 2, 4, 8 and 16mg/kg/i.p) for 6 days respectively. On 6th day, after administration of dexamethasone, the animals were sacrificed by cervical dislocation. Liver and aorta tissues were collected and washed thoroughly with normal saline. Liver weight and volume was measured, and stored in formalin for histopathological studies. Liver weights were measured by using electrical balance. Liver volumes were measured by following method.
Liver volume = Volume of saline displaced with liver in 100ml of saline.
Histopathological studies
A small piece of liver and aorta tissue were taken by using a sharp scalpel using little pressure. Tissues were fixed in 10% formalin for histopathological procedure. Sections were made 5μm thick, and stained with hemotoxylin and eosin stain (H&E stain). Section were observed under 45x Microscope and photographs were taken.
Statistical analysis
All the values are expressed in Mean±SEM. The statistical analyses were performed by using One-Way ANOVA followed by Scheffe multiple comparison post-hoc test. Statistical significance was assumed if p<0.05
Results
Treatment with dexamethasone induced fatty changes and increased cell size in a dose dependent manner. There was significant increase in liver weight and volume in all dexamethasone treated groups except in lowest dose of dexamethasone (0.5mg/kg) compared to control rats [Table/Fig-1].
Control rat liver showed normal hepatocytes, central vein and portal triad. Cytoplasm was clear to granular. Periportal (zone 1), midzone (zone 2) and centrilobular (zone 3) areas were normal [Table/Fig-2]. Treatment with 0.5 mg/kg/i.p dexamethasone did not induce any pathological changes in liver tissue. But, dexamethasone at 1 and 2mg/kg/i.p. has shown increased granularity and eosinophilic cytoplasm. Increase in hepatic cell size was observed in 4, 8 and 16mg/kg dexamethasone treated groups in a dose dependent fashion whereas the same was not seen in low doses of dexamethasone (0.5 and 1mg/kg). Treatment with higher doses of dexamethasone (4, 8 and 16mg/kg) resulted in fatty accumulation in liver and increased the size of hepatocytes. Cytoplasm was deeply eosinophilic with increased granularity, as shown in [Table/Fig-3,4,5].
Zone wise fatty changes seen in liver tissue treated with higher doses of dexamethasone. Initially, at 4mg/kg, fatty accumulation observed only in zone 3 (centrilobular area) and was spread in to zone 2 (mid zone) in 8mg/kg dexamethasone treatment. Dexamethasone at a dose of 16 mg/kg has induced severe fatty change in zone 1 (Periportal), 2 (Mid zone) & 3 (Centrilobular area) with formation of fatty cysts. Increase in size of hepatocytes was observed, nucleus was placed eccentrically [Table/Fig-5].
Dexamethasone treatment also induced dose dependent changes in aorta. Control rats showed normal aorta with tunica intima lined by normal endothelial cells, tunica media consisting of smooth muscle cells with elastic fibers and tunica adventitia is lined by loose connective tissue [Table/Fig-6]. Dexamethasone dose 0.5mg/kg/i.p. was not sufficient to induce any changes in aorta.
Mild thickening of tunica intima at 1mg/kg and tunica media at 2mg/ kg dose of dexamethasone was observed. Moderate and severe thickening of tunica media was observed in respective doses of 4 and 8mg/kg dexamethasone [Table/Fig-7,8]. Moderate to severe arteriosclerosis was seen in 4 and 8mg/kg dexamethasone treatment. Highest dose of dexamethasone (16mg/kg) induced severe thickening of tunica media with pink eosinophilic hyaline deposits and resulted in severe form of arteriosclerosis [Table/Fig-9]. Death (two rats) was observed at this particular dosage.
Discussion
Dexamethasone is a synthetic glucocorticoid which has a high affinity for the glucocorticoid receptor. It has been used to rapidly generate insulin resistance in rodents in a relatively short period of time [14]. There were numerous studies on dexamethasone induced insulin resistance, although the data is very scarce on steroid induced changes in liver and endothelium. Hence, the present study mainly focused to establish a new animal model in view of dexamethasone induced changes in liver and endothelium.
Glucocorticoid treatment is associated with abnormal fatty acid metabolism [15]. The present study reported that the treatment with higher doses of dexamethasone (4,8mg/kg/i.p.) produced fatty accumulation in liver parenchyma which resulted in hepatic steatosis. Steatosis is the first stage of non alcoholic fatty liver syndrome and is the hepatic manifestation of insulin resistance [16,17]. The mechanism responsible for development of steatosis might be due to break down of lipids into fatty acids (lipolysis), decreased sensitivity to insulin and deposition of fat in liver tissue [18]. Our study supports the same as there was increase in hepatic cell size due to the accumulation fat. However, the lower doses (0.5, 1mg/kg/i.p.) of dexamethasone did not shown any changes in liver size and histology, due to the inadequate concentration of drug or duration of treatment. Another small dose of (2mg/kg/i.p) dexamethasone showed only increase in hepatic cell size but not sufficient to induce steatotic changes in the liver.
Dexamethasone causes alteration in lipid metabolism, due to excessive fatty acid uptake or synthesis, decreased fatty acid β oxidation, or decreased secretion of VLDL particles which can contributes the fatty liver [3,5]. Prolonged deposition of fat in liver progresses to liver injury, hepatitis and irreversible deposition of fibrotic tissue. Decrease in functioning and cirrhosis of liver further leading to liver transplant [16,17]. Present study supports that the high dose (4,8mg/kg/i.p.) of dexamethasone induces hepatic steatosis which implicates insulin resistance and its complications which may lead to liver transplant.
Dose dependent changes in the endothelium were observed in the aorta except in the low dose (0.5mg/kg/i.p.) of dexamethasone compared to control rats. Dexamethasone can induce cardiovascular changes, hypertension which may be secondary complications of insulin resistance. Prolonged Dexamethasone treatment is known to be accompanied by serious side effects, like an impaired immune response, osteoporosis and an increased atherosclerotic events due to dyslipidemia [19]. Interestingly, in contrast to other studies [20], our study revealed that dexamethasone (4,8 and 16 mg/kg/i.p.) in higher doses has induced arteriosclerotic changes in endothelium. It was observed that the dose dependent thickening of middle and inner layers of endothelium such as tunica media and tunica intima which may lead to cardiovascular abnormalities. Thickening of endothelial layers like tunica media and adventitia was mainly due to hyaline deposits which may result in decrease in aortic compliance.
Few attempts have been made to identify the possible mechanism of dexamethasone induced cardiovascular complications, such as hypertension is mainly due to decreased aortic compliance as a consequence of impaired endothelium dependent relaxation and reduced synthesis of nitric oxide [11,21]. Hyperleptinemia induced by dexamethasone was also able to contribute to cardiovascular complications which correlate with insulin resistance [22]. Our study strongly supports the earlier work in which the dexamethasone reduces insulin sensitivity due to endothelial dysfunction and consequently develops other abnormalities like hypertension, fatty liver and alteration in the lipid metabolism [23]. In the present study, the endothelial changes in aorta may be due to insulin resistance induced by dexamethasone. But, the molecular mechanism of dexamethasone induced arteriosclerosis requires further exploration of work which is the main limitation of this study.
Liver weights and liver volumes of both control and dexamethasone treated rats
| Group | Liver weight | Liver volume |
|---|
| Control | 3.76±0.13*cdefg | 2.73±0.25*cdefg |
| 0.5 | 3.97±0.26*cdefg | 3.33±0.42*cdefg |
| 1 | 6.73±0.33*abdefg | 7.66±0.30*abdefg |
| 2 | 8.89±0.39*abcefg | 10.30±0.58*abcefg |
| 4 | 12.28±0.29*abcdfg | 14.15±0.67*abcdgf |
| 8 | 14.69±0.17*abcdeg | 16.51±0.29*abcdeg |
| 16 | 16.60±0.17*abcdef | 18.53±0.28*abcdef |
*= significance, a = control,b=0.5mg,c=1mg, d=2mg, e=4mg, f=8mg, g=16mg, p= <0.05
Histological sections of rat liver treated with Control (45X)
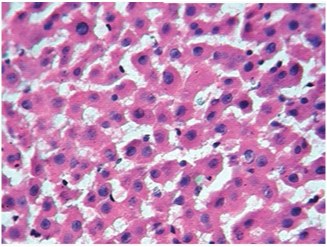
4mg Dexamethasone (45X).Arrow indicates fatty changes
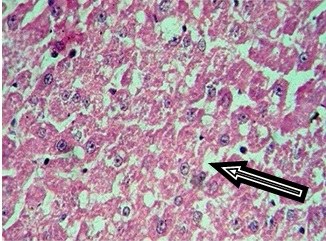
8mg Dexamethasone (45X).Arrow indicates fatty change
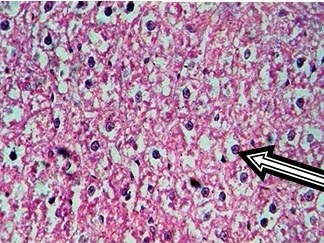
16mg Dexamethasone (45X).Arrow indicates fatty changes
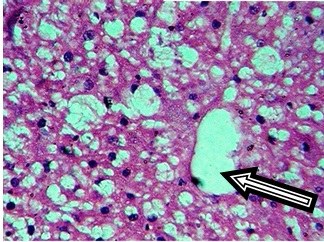
Histological sections of rat aorta treated with Control (45X)
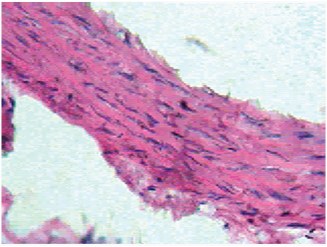
4mg Dexamethasone (45X).Moderate thickening, Moderate arteriosclerosis
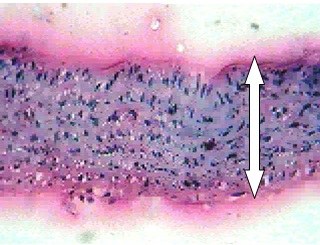
8mg Dexamethasone (45X).Severe thickening, and Severe arteriosclerosis
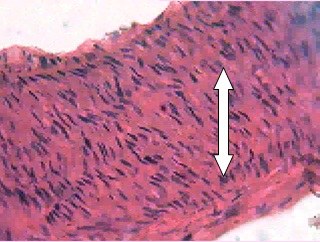
16mg Dexamethasone (45X).Severe thickening and severe arteriosclerosis
Conclusion
It is concluded that the acute high doses of dexamethasone (4 and 8mg/kg/i.p/6 days) induced insulin resistance and has resulted in fat accumulation in liver and induced sclerotic changes in endothelium. The present study established a new animal model to induce hepatic steatosis and arteriosclerosis by using dexamethasone in a relatively short period of time. This novel model can be used to develop insulin resistance in animals and to screen various medicinal plants and drugs in treating secondary complications of insulin resistance. It strongly suggests that acute high dose of dexamethasone can trigger the changes in liver parenchyma and endothelium. It could serve as a new animal model to study the secondary consequences of insulin resistance.
*= significance, a = control,b=0.5mg,c=1mg, d=2mg, e=4mg, f=8mg, g=16mg, p= <0.05
[1]. PS Bernard, LP Keith, Goodman & Gilman’s the pharmacological basis of therapeutics 2011 12th EditionNew YorkMcGraw Hill companies [Google Scholar]
[2]. A Stuart Morgan, M Sherlock, L Gathercole, G Lavery, C Lenaghan, J Bujalska, 11-Hydroxysteroid Dehydrogenase Type 1 Regulates Glucocorticoid-Induced Insulin Resistance in Skeletal MuscleDiabetes 2009 58(11):2506-15. [Google Scholar]
[3]. Patel R, Patel M, Tsai R, Lin V, Bookout AL, Zhang Y, LXR is required for glucocorticoid-induced hyperglycemia and hepatosteatosis in miceThe journal of clinical investigation 2011 121(1):431-41. [Google Scholar]
[4]. D Michael shanik, XU Yuping, skrha Jan, Insulin resitance and hyperinsulinemiaDiabetes care 2008 31(2):262-68. [Google Scholar]
[5]. RC Andrews, BR Walker, Glucocorticoids and insulin resistance: old hormones, new targetsClinical Science 1999 96(5):513-23. [Google Scholar]
[6]. A Giorgio, S Valerio Mattia, T Laura, C Marina, A Gloria, B Marco, Pathophysiology of dyslipidemia in Cushing’s syndromeNeuroendocrinology 2010 92(1):86-90. [Google Scholar]
[7]. Qi Dake, R Brian, Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolismAm J Physiol Endocrinol Metab 2007 292(3):E654-67. [Google Scholar]
[8]. P Letteron, N Brahimi Bourouina, MA Robin, A Moreau, G Feldmann, D Pessayre, Glucocorticoids inhibit mitochondrial matrix acyl-CoA dehydrogenases and fatty acid beta-oxidationAm J Physiol 1997 272(5):G1141-50. [Google Scholar]
[9]. K Girish, P Prasanth, K Minsuk, W Fang, L Vivan, H Nathania, Acute dexamethasone induced increase in cardiac lipoprotein lipase requires activation of both Akt and stress kinaseAm J Physiol Endocrinol Metab 2008 295(1):E137-47. [Google Scholar]
[10]. Defronzo RA, Ferrannini E, Insulin resistance A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular diseaseDiabetes care 1991 14(3):173-94. [Google Scholar]
[11]. K Yin, ZM Chu, LJ Beilin, Study of mechanisms of glucocorticoid hypertension in rats: endothelial related changes and their amelioration by dietary fish oilsBr J Pharmacol 1992 106(2):435-42. [Google Scholar]
[12]. M Korach Andre, J Gao, J Gounarides, R Deacon, A Islam, D Laurent, Relationship between visceral adiposity and intramyocellular lipid content in two models of insulin resistanceAm J Physiol Endocrinol Metab 2005 288(1):E106-16. [Google Scholar]
[13]. S Okumura, N Takeda, K Takami, K Yoshino, J Hattori, K Nakashima, Effects of troglitazone on dexamethasone-induced insulin resistance in ratsMetabolism 1998 47(3):351-54. [Google Scholar]
[14]. M Korach-André, J Gounarides, R Deacon, M Beil, D Sun, J Gao, D Laurent, Effect of dexamethasone on glucose tolerance and fat metabolism in a diet induced obesity mouse modelEndocrinology 2008 149(2):758-66. [Google Scholar]
[15]. Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RN, Leone TC, Dexamethasone induction of hypertension and diabetes is PPAR-α dependent in LDL receptor – null miceNature medicine 2003 9(8):1069-75. [Google Scholar]
[16]. G Marchesini, M Brizi, G Bianchi, S Tomassetti, E Bugianesi, M Lenzi, Non alcoholic fatty liver disease- A feature of the metabolic syndromeDiabetes 2001 50(8):1844-50. [Google Scholar]
[17]. LC David, GZ Damian, H Ryoko, DF Chad, EL Marie, JH Robert, Prenatal dexamethasone exposure potentiates diet-induced hepatosteatosis and decrease plasma IGF-1 in a sex specified fashionEndocrinology 2012 153(1):1-12. [Google Scholar]
[18]. Safaei N, Shomali T, Taherianfard M, Niacin ameliorates lipid Disturbances due to glucocorticoid administration in ratsIranian Journal of Basic Medical Sciences 2012 15(4):997-1002. [Google Scholar]
[19]. A Schepers, N Pires, D Eefting, M De Vries, JH Van Bockel, PH Quax, Short term dexamethasone treatment inhibits vein graft thickening in hypercholesterolemic ApoE3leiden transgenic miceJournal of vascular surgery 2006 43(4):809-15. [Google Scholar]
[20]. F Andreas, D Ralpha Agostino, H George, M Leena, P Russell Tracy, MH Steven, Chronic subclinical inflammation as part of insulin resistance syndrome the insulin resistance atherosclerosis study (IRAS)Circulation 2000 102(1):42-47. [Google Scholar]
[21]. W Thomas, W Klaus, CS Stephan, MS Petra, P Winfried, W Paulus, Down regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoidmediated hypertensionPNAS 1999 96(23):13357-62. [Google Scholar]
[22]. S Tahoora, T Mahnaz, F Mehdi, S Niloofar, Effect of niacin on hyperleptinemia and Ob gene mRNA over-expression in adipose tissue of dexamethasone treated ratsAmerican Journal of Pharmacology and Toxicology 2011 6(2):49-5. [Google Scholar]
[23]. C Severino, P Brizzi, A Solinas, G Secchi, M Maioli, G Tonolo, Low dose dexamethasone in the rat: a model to study insulin resistanceAm J Physiol Endocrinol Metab 2002 283(2):E367-73. [Google Scholar]