Introduction
Flavonoids are the largest group of naturally occurring poly phenolic compounds present almost in all parts of flowering plants. Flavones have reported to have interesting pharmacological action such as antinociceptive [1], anti-inflammatory [2] anti-oxidant [3], antihepatotoxic [4], anti-hypertensive [5], antiulcerogenic [6], antiallergic [7], anti-platelet [8] anti-microbial, anti-fungal, antiviral [9], anti-rhinovirus [10], antimalarial [11] and anticarcinogenic [12]. The combination of multiple pharmacological properties in a single nucleus is quite interesting.
Inflammation is a protective response intended to eliminate the initial cause of the cell injury as well as the necrotic cells and tissues resulting from insult. Inflammation is divided into two types, Acute inflammation is of relatively short duration, lasting from a minutes to days and it is characterized by fluid exudation. Chronic inflammation is of relatively long duration, lasting from days to years and associated with vascular proliferation and scarring [13]. One of the earliest therapeutic applications of flavonoids is in the treatment of some inflammatory diseases. The beneficial effects of flavonoids in rheumatoid arthritis [14] and in gingival inflammatory conditions [15] are some of the earliest reports in this regard. Subsequently, many flavonoid compounds have been reported to possess significant anti-inflammatory activity in several animal models of acute and chronic inflammation; taxifolin [16], gossypin [17] , hesperidin [18], naringin [19] and silymarin [20].
From the literature review it was concluded that certain dihydroxyflavones (3, 3'- dihydroxyflavone, 5, 6- dihydroxyflavone, 3, 7- dihydroxyflavone and 6, 3'- dihydroxyflavones) are proven to have significant anti-inflammatory activity [21]. With this view in minds four new dihydroxyflavone derivatives (2’,3’- dihydroxyflavone and 2’, 4’ -dihydroxyflavones, 5, 3’- dihydroxyflavone and 7, 3’ dihydroxyflavones) which were not subjected to any anti inflammatory studies have been selected. In a previous study done by the the authors, it has been proven that the selected dihydroxyflavones have potent antinociceptive action [22]. In the present study, the dihydroxyflavones were investigated for their effect on inflammation using carragenan induced paw oedema model.
Materials and Methods
2.1 Animals
Male wistar albino rats weighing between120-150 g were used for the study. The animals were housed in a controlled temperature (25°) with free access to pellet feed (Gold Mohar Ltd., Bangalore) & water and maintained under 12:12 h light:dark cycle. The experimental procedure was approved by the Institutional Animal Ethical Committee.(Sri Ramachandra Medical College and Research Institute).
2.2 Dihydroxyflavones
The dihydroxyflavones used in the present study;- 7, 3’ dihydroxyflavone, 2’,3’- dihydroxyflavone , 2’, 4’ -dihydroxyflavone and 5, 3’ dihrdroxy flavone were synthesized using standard procedures at Research Organics, Chennai, India. The authenticities of these compounds were done with melting points and UV method.
2.3 Toxicity studies
According to Organisation for economic cooperation and Development - OECD guidelines 423, acute toxicity studies were carried out for all the four dihydroxyflavones. After acclimization of the animal for 5 -7 days, study was conducted on healthy, young adult wistar albino male rats. The acute toxicity study was done by administering single dose 2000mg/kg of respective drugs. After drug administration, animals will be observed at 1, 2, 4 and 6 h on day 1 and then for the period 14 days for any clinical signs and mortality.
2.4 Carrageenan Induced Hind Paw Oedema in Rats
The anti-inflammatory effect was evaluated on the basis of carrageenan induced hind paw oedema method in rats [23]. Paw oedema was induced by subcutaneous injection of 0.1ml (1% solution) of Carrageenan into the plantar surface of the right hind paw of the rat. Selected dihydroxyflavones were administered in doses of 5, 10 and 50 mg/kg in different groups of animals, 30 min prior to carageenan injection. Doses of selected dihydroxyflavones were chosen based on the antinociceptive results reported earlier [22]. Diclofenac (10 mg/kg i.p.) was used as a standard antinflammatory drug which was administered 30 min prior to carrageenan injection. Animals were divided into 14 groups (n = 6) as follows
Group -- I - Control - treated with vehicle (carboxy methyl cellulose)
Group -- II - Standard drug – Diclofenac
Group – III to V - 2’,3’- dihydroxyflavone were administered in doses of 5, 10 and 50 mg/kg respectively.
Group – VI to VIII - 4’,3’- dihydroxyflavone were administered in doses of 5, 10 and 50 mg/kg respectively
Group – IX to XI - 5,3’- dihydroxyflavone were administered in doses of 5, 10 and 50 mg/kg respectively
Group – XII to XIV - 7,3’- dihydroxyflavone were administered in doses of 5, 10 and 50 mg/kg respectively
Paw diameters were measured immediately before the administration of the Carrageenan and thereafter every hour upto five hours using a plethysmograph. The results obtained with the selected DHF were compared with control group. The percentage inhibition of paw inflammation produced by selected DHF was calculated by using following formula:
% inhibition = C-T/ C X 100
C= Paw volume (ml) in vehicle treated group
T= Paw volume (ml) in drug treated group
Results
No mortality was observed up to the maximum dose of 2g/kg s.c for all four selected flavones.
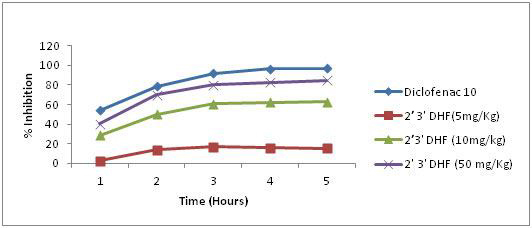
In vehicle (carboxy methyl cellulose) treated animal, carrageenan administration in the hind paw resulted in an increase in the paw volume 0.42 ± 0.01 ml after one hour. The paw volume was progressively increasing in these animals and a maximum of 0.76 ± 0.01 ml was noticed at third hour. This was declining afterwards and a volume of 0.66 ml was recorded after five hours. In diclofenac (10 mg/kg) pretreated animals, the volume of paw oedema remained very minimal during the entire period of observation. When compared to vehicle treatment a significant reduction in paw oedema was observed in the animals at all observation time. Diclofenac treated animal showed nearly 54% inhibition of inflammation at first hour and which was increased progressively to 97% at fifth hour. All the selected dihydroxy flavonoes showed significant dose and time dependent reduction in paw oedema. 2’, 3’ – dihydroxyflavones in doses of 5, 10 and 50 mg produced nearly 2.4%, 28.6%, 40.5% inhibition of inflammation during the first hour of observation and it increased progressively with time and reached a maximum of 15.2, 62.1 and 84.8% inhibition respectively during the fifth hour of observation period [Table/Fig-1].
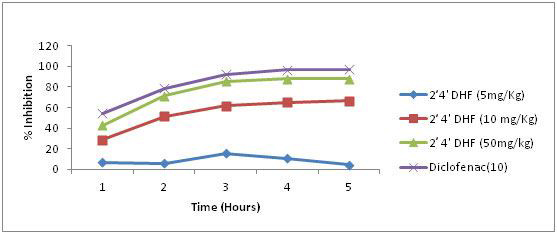
When compared to vehicle treatment 2’, 4’ dihydroxyflavone in a dose of 5 mg/kg produced a mild but significant reduction in the volume of paw oedema when measured at various time intervals. But in doses of 10 and 50 mg/kg a marked reduction in paw oedema was evidenced during the entire period of observation and showed a maximum percent inhibition of inflammation of 67 and 88 respectively during the fifth hour of observation [Table/Fig-2].
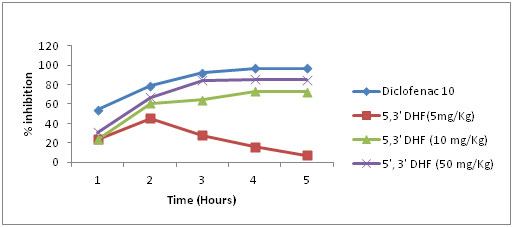
5, 3’ – Dihydroxyflavones in a dose of 5 mg/kg a maximum inhibition of 46% was noticed during the second hour of observation which started declining afterwards. However, in doses of 10 and 50 mg/kg it produced a progressive reduction in the paw oedema with a maximum inhibition of 73% and 85% respectively during the fourth hour of observation [Table/Fig-3].
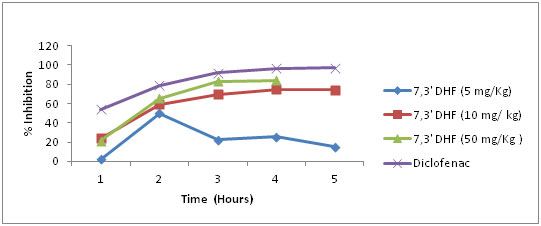
A significant dose and time dependent reduction in paw oedema was evident after treatment with 7, 3’ –DHF. In a dose of 5 mg/kg nearly 50 % inhibition was recorded at second hour of observation, but the response was gradually declining afterwards. However, a sustained inhibition in paw oedema was very obvious in doses of 10 and 50 mg/kg during all periods of observation. A maximum of 74% and 84% of inhibition were noticed in the above doses respectively when compared to 97% inhibition in Diclofenac treated animals during fifth hour of observation [Table/Fig-4].
Discussion
Inflammation is a very complex and regulated sequence of events acting as the primary line of protection by restricting the tissue damage [24]. The activities of a number of regulatory enzymes (e.g. protein tyrosine kinases, protein kinase C, phosphodiesterase, phospholipase A2, lipoxygenases, and cyclooxygenase) are essential to inflammation and the immune response. These enzymes are central to the activation of endothelial cells and numerous other specialized cells involved in inflammation, and it is with their inhibition of these enzymes [25] Carrageenan-induced inflammation is useful in determining orally active anti-inflammatory agents. Oedema formation due to carrageenan in the rat paw is a biphasic event. Initial phase is attributed to the release of histamine and serotonin. The oedema produced at the peak (180 min) is thought to be due to the release of kinin-like substances, especially of bradykinin. The second phase of oedema is due to the release of prostaglandins, protease and lysosome. The second phase is known to be sensitive to most clinically effective anti-inflammatory drugs [24].
The DHF selected for the present anti-inflammatory study exhibited the antinociceptive effect in inflammatory models of pain. Earlier studies on anti inflammatory activity of mono methoxy flavones, mono hydroxy flavones reveals that hydroxy derivatives of flavones were potent anti-inflammatory agents than their corresponding methoxy derivatives. Since the earlier studies indicated marked anti-inflammatory activity for monohydroxy flavones, the higher homologous series, the dihydroxyflavones were selected for the present study.
The compounds were screened for their effect on acute inflammation using carageenan induced paw oedema, a well-established animal model. Oedema represents the early phase of inflammation and the above method is the simplest and most widely used model for studying the anti-inflammatory activity of new compounds. Treatment with 2’,3’- dihydroxyflavone, 2’,4’- dihydroxyflavone, 5, 3’- dihrdroxy flavone and 7, 3’ dihydroxyflavone showed dose and time dependent reduction in paw oedema. All the four dihydroxyflavones produced nearly 81-88% inhibition of inflammation when employed in a dose of 50mg/kg.
Thus they can be considered as equally effective in their antiinflammatory activity. It is well known that Flavonoids are effective anti-inflammtory agent [26]. In an earlier study, monohydroxy flavones like 2’- hydroxy flavone and 4’ -hydroxy flavone showed less than 50% inhibition of paw oedema [27]. Treatment with 5, 6 -dihydroxyflavone, 3, 7- dihydroxyflavone and 6, 3’ dihydroxyflavone showed nearly 60% inhibition of inflammation [21].
All the four DHF subjected for present study produced nearly 81- 88% inhibition. However, 2’, 4’- DHF investigated in the present study inhibited rat paw oedema to the maximum extent of 88%. In general the anti-inflammatory efficacy of dihydroxyflavones appear to be greater than monohydroxy flavones.
Effect of 2,3’ dihydroxyflavones on rat paw edema in three different concentration
Effect of 2,4’ dihydroxyflavones on rat paw edema in three different concentrations
Effect of 5,3’ dihydroxyflavones on rat paw oedema in three different concentrations
Effect of 7, 3’ dihydroxyflavones on rat paw oedema in three different concentrations
Conclusion
The findings of the present study have shown all the selected Dihydroxyflavones; 2,3’- dihydroxyflavone , 2, 4’ -dihydroxyflavone, 5, 3’- dihydroxyflavone and 7, 3’ dihydroxyflavone have significant antinflammatory activity at the dose of 50mg/kg. The efficacy of anti inflammatory activity for the selected DHF was found to be in the order of 2, 4’ -dihydroxyflavones , 2, 3’ -dihydroxyflavones, 5,3’ -dihydroxyflavone and 7,3’ -dihydroxyflavones. The novel anti-inflammatory activity of dihydroxyflavones coupled with their antinociceptive property has enormous therapeutic applications. Future plan is to investigate the effect of selected dihydroxyflavones on the inflammatory mediators like Cyclooxygenase-1(COX-1), Cyclooxygenase-2(COX-2), Tumour necrosis factor (TNF-α) and Interlukin (IL-6).
[1]. P Thirugnanasambantham, S Viswanathan, V Krishnamoorty, C Mythirayee, S Ramachandran, L Kameswaran, Analgesic activity of certain flavones derivatives: A structure - activity studyJ Ethnopharmacol 1990 28:207 [Google Scholar]
[2]. R Arivudai Nambi, S Viswanathan, P Thirugnanasambantham, MK Reddy, ML Dewan, JS Sijher, Anti Inflammatory Activity Of Flavone and its Hydroxy Derivatives - A Structure Activity Study Indian J Pharm Sci 1996 58:18 [Google Scholar]
[3]. B Jayashree, A Alam, D Vijay Kumar, N Yogendra, Antioxidant and antibacterial activity of new 3- methylflavonesIndian. J Heterocy Chem 2010 19(3):237-40. [Google Scholar]
[4]. AR Tapas, DM Sakarkar, RB Kakden, Flavonoids as Nutraceuticals: A ReviewTrop J Pharm Res 2008 7(3):1089-99. [Google Scholar]
[5]. ESC Wu, TE Cole, TA Davidosn, JC Blosser, AR Borrelli, CR Kinsolving, Milgate TE, Parker RB. Flavones: Synthesis and antihypertensive activity of (3- phenylflavonoxy) propanolamines without beta.-adrenoceptor antagonismJ Med Chem 1987 30(5):788-92. [Google Scholar]
[6]. S Viswanathan, P Kulanthaivel, SK Nazimudeen, T Vinayagam, C Gopalakrishnan, L Kameswaran, The effect of apigenin 7, 4-di-O- methyl ether, a flavon from Andrographis paniculata on experimentally induced UlcersIndian J Pharm Sci 1981 43:159-61. [Google Scholar]
[7]. GH Quan, HS Chae, HH Song, KS Ahn, HK Lee, YH Kim, Anti-allergic flavones from Arthraxon hispidusChem Pharm Bull (Tokyo) 2013 61(9):920-26. [Google Scholar]
[8]. TC Wang, YL Chen, CC Tzeng, SS Liou, YL Chang, CM Teng, Antiplatelet α-Methylidene-γ- butyrolactones: Synthesis and evaluation of quinoline, flavone, and xanthone derivativesHelvetica Chimica Acta 1996 79(6):1620-26. [Google Scholar]
[9]. TP Cushnie, AJ Lamb, Anti-microbial activity of flavonoidsInt J Antimicrob Agents 2005 26(5):343-56. [Google Scholar]
[10]. C Conti, P Tomao, D Genovese, N Desideri, ML Stein, N Orsi, Mechanism of action of the Anti-rhino virus flavanoid 4',6- dicyanoflavanAntimicrob Agents Chemother 1992 36(1):95-99. [Google Scholar]
[11]. SS Lim, HS Kim, DU Leen, In vitro Antimalarial Activity of Flavonoids and ChalconesBull Korean Chem Soc 2007 28(12):2495-97. [Google Scholar]
[12]. Chahar Maheep K, Sharma Neelu, Dobhal Mahabeer P, Joshi Yogesh C, Flavonoid: A versatile sources of Anticancer drugsPharmacogn Rev 2011 5(9):1-12. [Google Scholar]
[13]. RS Cotran, V Kumar, SL Robbins, FJ Schoen, Robbins Pathologic Basis of Disease 2002 PhiladelphiaWB Saunders Company:33-35. [Google Scholar]
[14]. JM Rinehartn, Rheumatic fever; observations on the histogenesis, pathogenesis and use of ascorbic acid and bioflavonoid.Ann N Y Acad Sci 1955 61:684-99. [Google Scholar]
[15]. RI Carvel, VC Halperin, Therapeutic effect of water soluble bioflavonoids in gingival inflammatory conditionsOral Surg Oral Med and Oral Path 1961 14:847-55. [Google Scholar]
[16]. MBR Gupta, TN Bhalla, GP Gupta, CR Mitra, KP Bhargava, Antiinflammatory activity of taxifolinJap J Pharmacol 1971 21:377-82. [Google Scholar]
[17]. NS Parmar, MN Ghosh, Antiinflammatory activity of gossypin, a bioflavonoid isolated from Hibiscus Vitifolius LinnInd J pharmacol 1978 10:277-93. [Google Scholar]
[18]. F Shahidi, Z Yang, ZO Saleemi, Natural flavonoids as stabilizersJ Food Lipids 1998 1:69-75. [Google Scholar]
[19]. BH Havsteen, The Biochemistry and medical significance of the FlavonoidsPharmacol Ther 2002 96:67-202. [Google Scholar]
[20]. OP Gupta, S Singh, S Bani, N Sharma, S Malhotra, BD Gupta, Antiinflammatory and anti arthritic activities of silymarin acting through inhibition of 5-lipoxygenasePhytomed 2000 1:21-24. [Google Scholar]
[21]. K Vidyalakshmi, P Kamalakannan, S Viswanathan, S Ramaswamy, Antiinflammatory effect of certain dihydroxyflavones and the mechanisms involvedAntiinflamm Antiallergy Agents Med Chem 2012 11(3):253-61. [Google Scholar]
[22]. S Umamaheswari, S Viswanathan, S Sathiyasekaran, S Parvathavarthini, S Ramaswamy, Antinociceptive activity of certain dihydroxyflavonesIndian Journal of Pharmaceutical Science 2006 68(6):749-53. [Google Scholar]
[23]. CA Winter, EA Risley, CW Nusss, Carageenan induced oedema in hind paw of the rats; an assay for anti inflammatory drugsProc Soc Exp Biol Med 1962 111:544-7. [Google Scholar]
[24]. O Olajide, JM Makinde, SO Awe, Effect of the aqueous extract of Bridelia ferruginea stem. bark on carrageenan-induced ooedema and granuloma tissuen formation in rats and miceJ Ethnopharmacol 1999 66:113-17. [Google Scholar]
[25]. Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K, Mechanism of Action of Flavonoids as Anti-inflammatory Agents: A Review.Inflammation & Allergy - Drug Targets 2009 8:229-35. [Google Scholar]
[26]. M Joo, HS Kim, TH Kwon, A Palikhe, TS Zaw, JH Jeong, Antiinflammatory Effects of Flavonoids on TNBS-induced Colitis of RatsKorean J Physiol Pharmacol 2015 19(1):43-50. [Google Scholar]
[27]. NS Muthiah, S Viswanathan, P Thirugnanasambantham, MK Reddy, V Vijayasekaran, Anti inflammatory activity of flavone and its methoxy derivatives – A structure Activity StudyIndian J Pharm Sci. 1993 55:180-83. [Google Scholar]