Papillon-Lefevre Syndrome (PLS) is a rare inherited autosomal-recessive condition with one-third of the patients’ showing consanguinity of the parents. Lesions are characterised by palmar-plantar hyperkeratosis and hyperhidrosis. Early onset of periodonditis, severe periodontal destruction in both primary and permanent dentitions, and calcification of the duramater form the three important features of this disease. Here, we present a case of a 14-year-old female who presented to the Department of Oral Medicine and Radiology with a complaint of mobility of the teeth since four months. Oral examination of the patient showed generalised mobility of the teeth. General physical examination of the patient showed dry scaly skin on dorsum of bilateral feet, hands, and knee. The patient had familial history positive for consanguinity. The patient was medically diagnosed as positive for PLS. The patients with PLS show combination of dermatological and dental lesions and it requires the dentist to assume a more prominent role in early treatment and rehabilitation. There is a need for symbiotic and synergetic approach between the two specialties for effective management of this rare disease.
Case Report
A 14-year-old female patient came with a chief complaint of loosening of multiple teeth in the upper and lower jaws. The patient initially noticed loosening of teeth one year ago, which aggravated in the past four months. The patient apparently also had early exfoliation of her primary teeth. Past history revealed that dry scaly skin started at the age of six, as noticed by her family, over the feet which later extended to bilateral hands and knees. The family history was positive for consanguinity of parents. The patient has two siblings who were clinically unaffected.
General and extra-oral examination
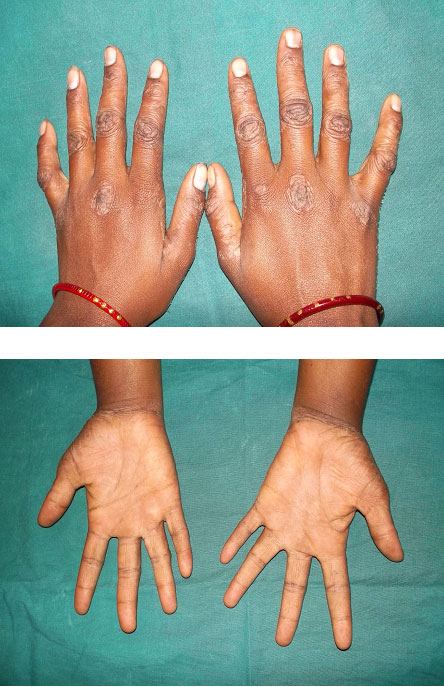
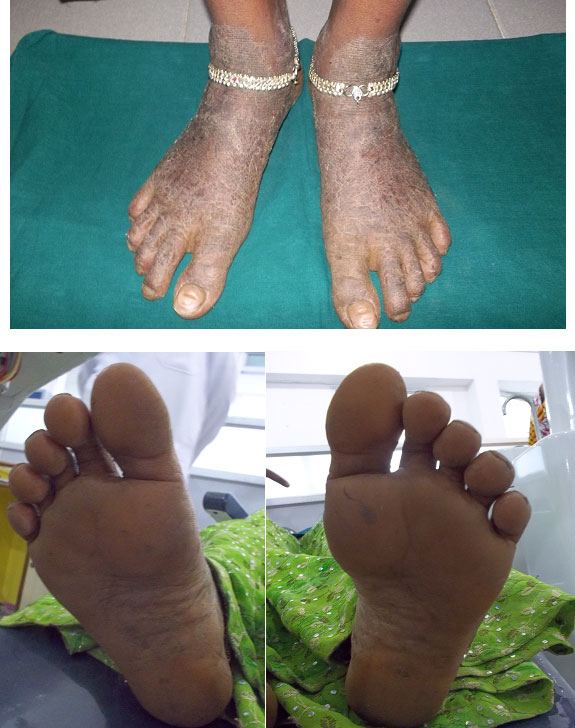
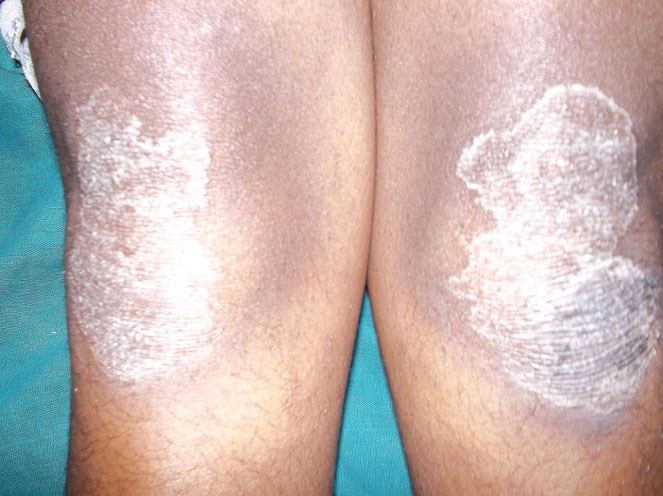
General physical examination revealed well demarcated lesions of dry scaly skin present bilaterally on knee, dorsum surface of hands and feet [Table/Fig-1,2,3]. The lesions extended 1 cm above the ankle joint whereas it involved metacarpal and interphalangeal joints in the hands. These keratotic lesions were dry, scaly, and rough on palpation. She was receiving treatment for the same for the past seven years. The patient had right corneal opacity with loss of vision. A pustular lesion was also noted on the left lower eye lid. The patient was well oriented and showed independent ability to understand and communicate. There was absence of axillary hair. Bilateral submandibular lymphnodes were enlarged (2 cm X 2 cm), mobile, firm in consistency and non-tender [Table/Fig-4].
Hyperkeratotic soles and feet
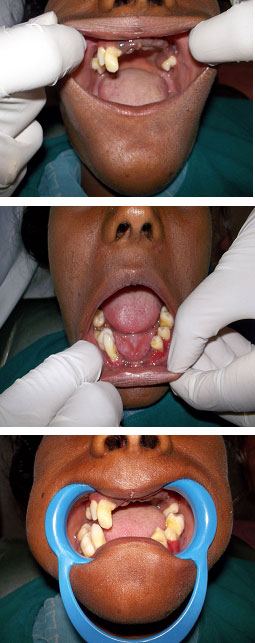
Intraoral examination revealed early exfoliation of upper incisors, premolars, and molars (#11, #14, #15, #16, #21, # 22, #23, #24, #25, and #26) and lower incisors and premolars (#31, #32, #33, #42, #44, and #45). There was pathological migration of #43, #46, and #47 noted. The gingiva surrounding the tooth was inflamed, oedematous, and soft whereas the mucosa covering the edentulous regions appeared normal. There was mild accumulation of plaque around the teeth. The teeth exhibited generalised mobility grade III with formation of periodontal pockets and furcation involvement in teeth. Trauma from occlusion was absent. According to the American College of Prosthodontists Prostodontic Diagnostic Index (ACPPDI), the edentulism and prosthetic needs are as follows class II Classification characterized by the early onset of systemic disease interaction and by specific patient management and lifestyle considerations.
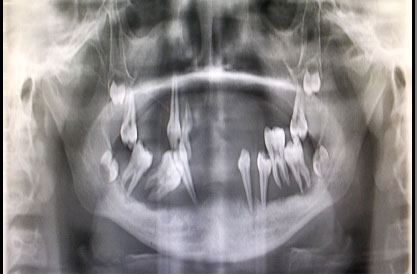
Radiographic finding
Panoramic view radiograph showed the classical “floating-in-air” appearance with generalized horizontal and vertical bone loss of all the existing teeth [Table/Fig-5].
Panoramic radiograhic appearance of the maxilla and mandible
Laboratory examinations
Routine haematological examination revealed elevated ESR of 40 mm/h, decreased random blood sugar was 63 mg/dl, and elevated total leukocyte count was 12350. Alkaline phosphatase levels were significantly reduced.
Recapitulation of the clinical features, family history, and investigations confirmed it as Papillon-Lefevre syndrome (PLS). The differential diagnoses of Haim-Munk syndrome and hypophosphatasia were considered.
As for the treatment plan, importance of oral hygiene was stressed and enforcement of oral hygiene habits was advised. Extractions of painful and mobile teeth were performed. Patient was advised for total extraction and complete oral rehabilitation in the near future. The patient was advised to continue her treatment with dermatologist for further management which included topical keratolytic 5% salicylic acid in combination with 10% urea treatment. A photochemotherapy acitretin (10 mg/day) (PUVA) were initiated. Nickles et al., have reported mixed results in its management. They have had some success in combination of mechanical and antibiotic treatment, selective extraction of involved teeth, maintenance of oral hygiene, continued maintenance therapy, and monitoring and treatment of microbial infection by Aggregatibacter actinomycetemcomitans. They considered treatment of PLS as high risk [1]. The patient was followed up after two weeks for the total extraction of hopeless teeth.
Discussion
PLS syndrome is an autosomal-recessive inherited disorder showing dermatological and dental lesions, with calcification of the duramater forming the third component.
The cause of development of these lesions is attributed to three main factors namely genetic, immunologic, microbiologic [2]. The genetic factor which is due to impairment leading to mutations of cathepsin C gene (CTSC) in the region of chromosome 11q14-21 is responsible for causing this disease [3]. CTSC messages which are expressed in high levels in lungs, kidney, placenta, polymorphonuclear leukocytes, and alveolar macrophages [4] encodes the cathepsin C protein, which is an oligomeric enzyme consisting of 4 subunits [5,6]. Cathepsin C also known as dipeptidyl peptidase is a cysteine proteinase and is proposed to play roles in epithelial differentiation and desquamation. [7] A total of 75 mutations are reported in CTSC gene [8]. Among these, mutations occurring in exon _5–7 are missense and nonsense mutations and are proposed to alter the enzymatic activity of cathepsin C gene [9]. Role of immunologic component in the causation of PLS is complex. Immunological factor responsible for PLS mainly depends on the deterioration of neutrophil chemotaxis, phagocytosis, bactericidal capabilities, decreased cell migration, lymphocytic response, and monocytic activity [10,11]. Impairment in natural killer cells cytotoxicity has been implicated in the development of PLS [11]. Loss of activity of CTSC gene and consequent inactivity of neutrophil serine proteinases may deregulate local polymorphonuclear response and cause severe tissue damage [12–14]. However, in a recent paper by Sorensen et al., it has been proposed that neutrophil serine proteinases are not indispensible for human immunoprotection [15]. They further suggested that CTSC protects degradation of serine proteinases only in mature immune cell subsets and not in progenitor immune cells.
Loss of activity of cathepsin C has also been linked with lack of immunomodulatory and microbial function of LL-37 in infected periodontum [16]. In one of the study investigators desired to understand if proinflammatory cytokines levels are elevated in blood samples of PLS patients [17]. However, they could not find any statistically significant rise in the level of cytokines. Apart from the role of gene mutation and deregulation of several immunologic factors in the cause and development of PLS, low level of anti-inflammatory fatty acids has also been reported in the patients with PLS [18]. It is observed that various periodontologic pathogens are known to proliferate in PLS patients. Microbial flora in subgingival plaque is very complex. Albandar conducted a detailed study of PLS microbiota and detected 12 bacterial species [19]. Robertson et al., found periodontologic pathogens like A. actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in the subgingival plaque of PLS patients [20]. Presence of A. actinomycetumcomitans in the periodontal pockets is known to act as triggering factor for PLS development [14].
In large number of cases of PLS, it has been described in the scientific literature that consanguinity is a potential risk factor in the development of PLS. Recently, a paper by Shah et al., described two cases of PLS who were born of consanguineously married parents [21]. Another paper by Valeshabad et al., described six cases of PLS in the same family [22]. Palmoplantar hyperkeratosis was detected in all of the cases, and two cases had painful lesions on the soles. The lesions and abscesses in the internal organs are frequently associated with PLS. For the first time, Kanthimathinathan et al reported presence of brain abscess in a child with PLS [23]. Presence of liver abscess has also been reported in one of the two siblings who were born of consanguineously married parents [24]. Morgan et al., described a case of 5-year-old patient of PLS who developed a renal mass [25]. Therefore, it may be concluded that PLS is often present with lesions in different internal body parts and the complications of PLS are frequently observed in first generation of consanguineously married couples.
Provisional diagnoses of aggressive periodontitis type 1 and palmoplantar hyperkeratosis and differential diagnoses of Haim-Munk syndrome and hypophosphatasia were initially considered for the patient. We confirmed the diagnosis as PLS on the basis of fulfilling classical description of the syndrome. The familial history of the patient was consistent with consanguinity. On physical examination, the patient showed characteristic diffuse skin lesions in the knees, dorsum of hands and feet. The lesions were dry, scaly, and rough. The dental history of the patient was compatible with classical symptoms namely early exfoliation of deciduous teeth followed by beginning of early exfoliation of most of the permanent teeth. The negative finding was absence of calcification of duramater when observed in PA radiograph of the skull. However, we could not confirm mutation of cathepsin C gene owing to the poor economical condition of the patient.
Keratotic changes are often associated with other hereditary keratodermas like Unna-Thost and Mal De Meleda. They are heterogeneous group of disorders with overlapping clinical features [26]. However, the dental findings are confined to PLS and absent in the above two conditions.
The treatment for PLS is more palliative in nature. Oral retinoids are used in the treatment of both keratoderma and the periodontitis. Retinoids are more beneficial when they are administered before the eruption of permanent teeth. Professional oral prophylaxis and antibiotics are used for the control of periodontitis. If the teeth show marked mobility, early extraction remains the treatment of choice to prevent further bone resorption [27]. A firm bony base enables construction of good quality artificial dentures in the future. There is a need for collaborative effort between the dentist and the deramatologist for better management of this condition. Early detection by dermatologist and immediate referral to dentist can help in control of periodontitis and suitable oral rehabilitation. Similarly early referral to dermatologist can aid in initiation of early treatment with retinoids to help prevent further progression of skin lesions.
Conclusion
We have discussed about a 14 year Old adolescent female diagnosed of Papillon-Lefevre syndrome. Thus both the dermatologist and dental professional can help to save the permanent dentition if they diagnose this disease during childhood. Osseointegrated implants are an option for the future and can have a great impact psycho socially by restoring esthetics as well as function.
[1]. Nickles K, Schacher B, Ratka-Kruger P, Krebs M, Eickholz P, Long-term results after treatment of periodontitis in patients with Papillon-Lefevre syndrome: success and failureJ Clin Periodontol 2013 40(8):789-98.doi: 101111/jcpe12120 [Google Scholar]
[2]. Hattab FN, Rawashdeh MA, Yassin OM, al-Momani AS, al-Ubosi MM, Papillon-Lefevre syndrome: a review of the literature and report of 4 casesJ Periodontol 1995 66(5):413-20. [Google Scholar]
[3]. Hart TC, Hart PS, Bowden DW, Michalec MD, Callison SA, Walker SJ, Mutations of the cathepsin C gene are responsible for Papillon-Lefevre syndromeJ Med Genet 1999 36(12):881-87. [Google Scholar]
[4]. Rao NV, Rao GV, Hoidal JR, Human dipeptidyl-peptidase I. Gene characterization, localization, and expressionJ Biol Chem 1997 272(15):10260-65. [Google Scholar]
[5]. Dolenc I, Turk B, Pungercic G, Ritonja A, Turk V, Oligomeric structure and substrate induced inhibition of human cathepsin CJ Biol Chem 1995 270(37):21626-31. [Google Scholar]
[6]. Paris A, Strukelj B, Pungercar J, Renko M, Dolenc I, Turk V, Molecular cloning and sequence analysis of human preprocathepsin CFEBS Lett 1995 369(2-3):326-30. [Google Scholar]
[7]. Toomes C, James J, Wood AJ, Wu CL, McCormick D, Lench N, Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosisNat Genet 1999 23(4):421-24. [Google Scholar]
[8]. Nagy N, Valyi P, Csoma Z, Sulak A, Tripolszki K, Farkas K, CTSC and Papillon-Lefevre syndrome: detection of recurrent mutations in Hungarian patients, a review of published variants and database updateMol Genet Genomic Med 2014 2(3):217-28.doi: 101002/mgg361 Epub 2014 Feb 11 [Google Scholar]
[9]. Turk D, Janjic V, Stern I, Podobnik M, Lamba D, Dahl SW, Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteasesEMBO J 2001 20(23):6570-82. [Google Scholar]
[10]. Liu R, Cao C, Meng H, Tang Z, Leukocyte functions in 2 cases of Papillon-Lefevre syndromeJ Clin Periodontol 2000 27(1):69-73. [Google Scholar]
[11]. Lundgren T, Parhar RS, Renvert S, Tatakis DN, Impaired cytotoxicity in Papillon-Lefevre syndromeJ Dent Res 2005 84(5):414-17. [Google Scholar]
[12]. Bhavsar MV, Brahmbhatt NA, Sahayata VN, Bhavsar NV, Papillon-Lefevre syndrome: Case series and review of literatureJ Indian Soc Periodontol 2013 17(6):806-11.doi: 104103/0972-124X124530 [Google Scholar]
[13]. Ryu OH, Choi SJ, Firatli E, Choi SW, Hart PS, Shen RF, Proteolysis of macrophage inflammatory protein-1alpha isoforms LD78beta and LD78 alpha by neutrophil-derived serine proteasesJ Biol Chem 2005 280(17):17415-21.Epub 2005 Feb 22 [Google Scholar]
[14]. de Haar SF, Hiemstra PS, van Steenbergen MT, Everts V, Beertsen W, Role of polymorphonuclear leukocyte-derived serine proteinases in defense against Actinobacillus actinomycetemcomitansInfect Immun 2006 74(9):5284-91. [Google Scholar]
[15]. Sorensen OE, Clemmensen SN, Dahl SL, Ostergaard O, Heegaard NH, Glenthoj A, Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defensesJ Clin Invest 2014 124(10):4539-48.doi: 101172/JCI76009 Epub 2014 Sep 17 [Google Scholar]
[16]. Eick S, Puklo M, Adamowicz K, Kantyka T, Hiemstra P, Stennicke H, Lack of cathelicidin processing in Papillon-Lefevre syndrome patients reveals essential role of LL-37 in periodontal homeostasisOrphanet J Rare Dis 2014 9:148doi: 101186/s13023-014-0148-y [Google Scholar]
[17]. Sadik CD, Noack B, Schacher B, Pfeilschifter J, Muhl H, Eickholz P, Cytokine production by leukocytes of Papillon-Lefevre syndrome patients in whole blood culturesClin Oral Invest 2012 16(2):591-97.doi: 101007/s00784-011-0532-0 Epub 2011 Mar 5 [Google Scholar]
[18]. Bullon P, Morillo JM, Thakker N, Veeramachaneni R, Quiles JL, Ramirez-Tortosa MC, Confirmation of oxidative stress and fatty acid disturbances in two further Papillon-Lefevre syndrome families with identification of a new mutationJ Eur Acad Dermatol Venereol 2014 28(8):1049-56.doi: 101111/jdv12265 Epub 2013 Sep 3 [Google Scholar]
[19]. Albandar JM, Khattab R, Monem F, Barbuto SM, Paster BJ, The subgingival microbiota of Papillon-Lefevre syndromeJ Periodontol 2012 83(7):902-08.doi: 101902/jop2011110450 Epub 2011 Dec 5 [Google Scholar]
[20]. Robertson KL, Drucker DB, James J, Blinkhorn AS, Hamlet S, Bird PS, A microbiological study of Papillon-Lefevre syndrome in two patientsJ Clin Pathol 2001 54(5):371-76. [Google Scholar]
[21]. Shah AF, Tangade P, Agarwal S, Papillon-Lefevre syndrome: Reporting consanguinity as a risk factorSaudi Dent J 2014 26(3):126-31.doi: 101016/jsdentj201402004 Epub 2014 Apr 19 [Google Scholar]
[22]. Kord Valeshabad A, Mazidi A, Kord Valeshabad R, Imani E, Kord H, Koohkan M, Papillon-Lefevre syndrome: a series of six cases in the same familyISRN Dermatol 2012 2012:139104doi: 105402/2012/139104 Epub 2012 Dec 3 [Google Scholar]
[23]. Kanthimathinathan HK, Browne F, Ramirez R, McKaig S, Debelle G, Martin J, Multiple cerebral abscesses in Papillon-Lefevre syndromeChilds Nerv Syst 2013 29(8):1227-9.doi: 101007/s00381-013-2152-52 Epub 2013 May 18 [Google Scholar]
[24]. Mercy P, Singh A, Ghorpade AK, Das M, Upadhyay A, Papillon-Lefevre syndrome: two siblings, one developing liver abscessIndian J Dermatol 2013 58(5):410doi: 104103/0019-5154117361 [Google Scholar]
[25]. Morgan RD, Hannon E, Lakhoo K, Renal abscess in Papillion-Lefevre syndromePediatr Surg Int 2011 27(12):1381-3.doi: 101007/s00383-011-2931-33 Epub 2011 May 19 [Google Scholar]
[26]. Lestringant GG, Hadi SM, Qayed KI, Blayney BJ, Mal de Meleda: recessive transgressive palmoplantar keratoderma with three unusual facultative featuresDermatology 1992 184(1):78-82. [Google Scholar]
[27]. Janjua SA, Khachemoune A, Papillon-Lefevre syndrome: case report and review of the literatureDermatol Online J 2004 10(1):13 [Google Scholar]