Background
Pain affects millions of people worldwide, opioid analgesics have been used for chronic painful conditions. Due to their adverse effects, safer alternatives would be beneficial. Terminalia chebula, with proven analgesic action has been evaluated in the hot air pain model for its analgesic activity.
Aim
To evaluate analgesic activity and safety of single oral dose of Terminalia chebula using hot air pain model in healthy human participants.
Setting and Design
Randomized, Double blind, Placebo controlled, Cross over study.
Materials and Methods
After taking written informed consent to IEC approved protocol, 12 healthy human participants were randomized to receive either single oral dose of two capsules of Terminalia chebula 500 mg each or identical placebo capsules in a double blinded manner. Thermal pain was assessed using hot air analgesiometer, to deliver thermal pain stimulus. Mean Pain Threshold time and Mean Pain Tolerance time measured in seconds at baseline and 180 minutes post drug. A washout period of two weeks was given for cross-over between the two treatments.
Results
Terminalia chebula significantly increased mean pain threshold and tolerance time compared to baseline and placebo. Mean pain threshold time increased from 34.06±2.63 seconds to 41.00±2.99 seconds (p<0.001) and mean pain tolerance time increased from 49.67± 3.72 seconds to 57.30±3.07 seconds (p<0.001). The increase in mean percentage change for pain threshold time is 20.42% (p<0.001) and for pain tolerance time is 17.50% (p<0.001).
Conclusion
In the present study, Terminalia chebula significantly increased Pain Threshold time and Pain Tolerance time compared to Placebo. Study medications were well tolerated.
Introduction
Pain is defined as an unpleasant sensory and emotional experience associated with or without tissue damage [1]. The sensation of pain is triggered by stimuli from different sources like thermal, electrical and chemical through peripheral receptors at sufficient thresholds to cause damage [2]. Analgesics decrease the sensation of pain by increasing the threshold of pain to external stimuli with no change in the level of consciousness [3]. Traditionally non-steroidal anti inflammatory drugs (NSAID) have been the main stay of treatment in chronic painful conditions. Gastrointestinal toxicity manifested as ulcers, haemorrhage and perforation are present in 50% of NSAIDs users and 5.4% develop a more serious event requiring hospitalization due to its frequent use [4]. Further hypertension and renal damage are also associated with prolonged NSAIDs therapy, even when used in their therapeutic doses [5]. Opioids analgesics are other important group of drugs used in pain management with very good analgesic activity, but they are associated with a lot of side effects ranging from drowsiness, constipation, nausea, vomiting, dry mouth, itching, dependence potential and respiratory depression [6]. Hence there is a constant need for alternative analgesics with few side effects as compared to the traditional NSAID [3]. Due to these drawbacks intense research is going on to develop analgesics with minimal side effects in allopathic as well as complementary and alternative systems of medicine [7,8]. Established experimentally induced pain models can be used as means in early drug development to evaluate novel drugs for their analgesic activity in healthy participants under controlled conditions [9]. Many experimentally induced pain models like thermal pain, electrical, chemical and mechanical, etc, are used for evaluating new drugs [10]. There are various thermal pain models used conventionally, like cold pressor, contact heat, laser and radiant heat methods. Each of these methods are accompanied by their own limitations regarding evaluation of pain in humans [9] Sunil et al., validated and standardized hot air pain model, a thermal pain model for evaluation of analgesics in humans [11]. In a study by Nalini et al., have evaluated the analgesic activity of Withania somnifera in hot air pain model [12]. In this study we have used the hot air pain model, for testing the analgesic activity of newer drugs. Pain is the most common reason for people to use complementary and alternative medicine [13]. A safe and efficacious complementary treatment for pain alleviation would be beneficial for patients needing relief from chronic pain syndroms. Terminalia chebula belonging to the family combretaceae, commonly called as Black myrobalan, Ink tree (or) Chebulic myrobalan. It has been shown that this herbal remedy has anti-inflammatory and analgesic effect. Preliminary studies have indicated anti-inflammatory activity for the aqueous extracts of fruits of Terminalia chebula and that they could inhibit COX-1, COX-2, 5-LOX, TNF-alpha and down regulate NFkB [14]. The present study was thus designed to evaluate the analgesic efficacy of standardized aqueous extract of Terminalia chebula in experimentally induced hot air pain model in healthy human participants.
Materials and Methods
This is a randomized, double blind, placebo controlled, cross over study assessing the safety and efficacy of Terminalia chebula, approved by Institutional Ethics Committee and all the participants have given prior written informed consent.
Hot Air Analgesiometer
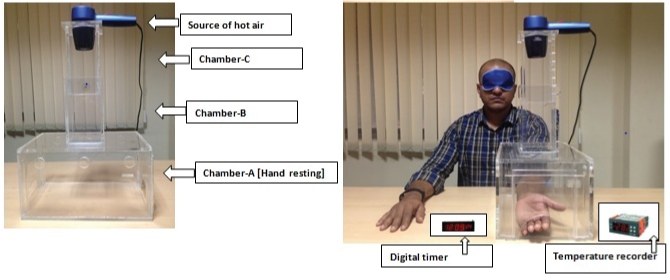
In the present study a hot air analgesiometer was used to deliver the thermal pain stimulus that has been developed, validated and described in detail elsewhere [11]. It delivers variable, quantifiable, and reproducible heat stimulus to induce thermal pain on the volar surface of subjects’ forearm as shown in [Table/Fig-1].
Study medication
Terminalia chebula capsules - 2 capsules of 500 mg (1000 mg) as a single dose [15]. Available as an highly standardized aqueous extract of the edible fruits of Terminalia chebula by high performance liquid chromatography containing not less than 15% of Chebulinic acid, not less than 10% of Chebulagic acid and not less than 15% of other low molecular weight hydrolysable tannins). Identical matching placebo was used containing microcrystalline cellulose.
Sample Selection
Inclusion criteria
Healthy male participants aged between 18-45 years, with a normal body mass index willing to take part in study by strict adherence to protocol and study related procedures were enrolled. The participants having pain tolerance time between 30 seconds and 180 seconds with short level and high heat intensity will be included into the study [11]. No history of diabetes, hypertension, GIT disorders.
Exclusion criteria
Participants who are not co-operative, smokers, alcoholics, drug abusers or any prior wounds or fractures on the tested extremity. Any significant abnormality identified on physical examination or laboratory tests. Participants using other non-steroidal anti-inflammatory drugs in the past two weeks and having documented history of treatment with amphetamines, cocaine, sympathomimetics, systemic glucocorticoids, anabolic steroids, will be not included in the study. The participants having pain threshold time less than 30 seconds and/or more than 180 seconds with short level and high heat intensity will be excluded from the study. Participants with a history of hypersensitivity to the study drug and who are on any other investigational drugs at the time of enrollment, or within 3 months prior to this study.
Materials and Methods
The duration of the study was three months. Written informed consent for participating in the study was taken from the participants. They were explained in detail about the test procedure. Initially the participants were screened for eligibility into the study. As a part of screening, medical history of the participant was taken, physical examination, clinical examination and laboratory tests were done. The subjects were trained on two separate occasions on the study procedure prior to participation to get accustomed with instrument and reduce variability. The participants were instructed to come in fasting state at 8:00 AM on study day after an optimum overnight sleep. Participants were housed in a temperature and humidity controlled room for 30 minutes, after which baseline vital parameters were recorded. Participants were helped by investigator to keep their non dominant hand exposing the volar surface of forearm into the lower chamber-A, and blindfolded just before delivering heat stimulus. Without prior notice, Hot air analgesiometer was turned on by the investigator and hot air was blown by adjusting the height of chamber B to the short level of 36.5 cm (level 1) and blowing air at high speed. The participants were instructed to indicate as soon as they perceive the heat sensation as painful (pain threshold), by raising their index finger, and to pull his forearm away from the chamber when they perceived the heat to be unbearable (pain tolerance time) and immediately the hair drier was to be turned off. On each occasion, the test procedure with heat stimulus was repeated thrice to record pain threshold time and pain tolerance time with inter-stimulus interval of five minutes [11]. The mean of the three measurements was determined. At 9.00 AM volunteers were served light breakfast and at 9.30 AM either two capsules of Terminalia chebula 500mg or identical looking placebo capsules with 200ml of water will be given as per computer generated random numbers (simple randomization). Treatment allocation was not known to participant or to the investigator. After taking the drug the participant was asked to sit upright in the chair. After 180 minutes post drug, test procedure was again repeated three times with an inter stimulus interval of five minutes. The pain threshold time and pain tolerance time readings will be noted and used for analysis. Participants were asked to report any side effects during the study. After two week wash out period the second medication was administered according to the randomization sequence and same procedures were repeated.
Ethics
All the procedures followed were in accordance with the ethical standards of the Nizam’s institute of medical sciences Institutional Ethics Committee (NIEC) on human experimentation and with the revised Helsinki Declaration. The registration number for this study is CTRI/2014/10/005106.
Statistical Analysis
All the data are presented as Mean±SD. Primary parameters, pain threshold and tolerance time is recorded in seconds. The baseline and post drug values within group were analysed by paired t-test. Unpaired t-test was used to compare in between groups. A power of 80% with a significance value of p<0.05 was set by using Graph pad prism software version 4.
Results
A total of 14 participants were screened and 12 completed the study. Two were excluded because their pain threshold time was less than 30 seconds. Twelve participants were assigned according to cross over design (participants randomized to Terminalia chebula 500mg initially were crossed over to Placebo group and participants randomized to Placebo group initially were crossed over to Terminalia chebula 500mg after washout period. Baseline demographic characteristics were comparable and homogenous in study groups as shown in [Table/Fig-2]. Terminalia chebula significantly increased mean pain threshold time and mean pain tolerance time compared to baseline and placebo as shown in [Table/Fig-3].
Terminalia chebula significantly increased mean percentage change in pain threshold time and pain tolerance time compared to placebo. The increase in mean percentage change for pain threshold time is 20.42% (p<0.001) and for pain tolerance time is 17.50 % (p<0.001).
Compliance was assured because of supervised administration of drugs. Both the medications were well tolerated and no subject discontinued the study because of adverse events. All safety lab parameters (haemogram, renal function tests, hepatic function tests, ECG, Random blood sugar) were repeated after the test procedure and found to be within normal limits.
Discussion
In the present study, Terminalia chebula 1000 mg was found to have significant increase in pain threshold and pain tolerance time in hot air pain model. Inflammation is a normal protective mechanism adopted by the body to counter offending stimuli, but if not properly treated may result to a more damage with exuberance to create chronic inflammation [16] and other diseases [17]. Inflammation and pain are linked by cyclooxygenase (COX) enzymes especially COX2 which help in the synthesis of prostaglandins (PGs) precisely PGE2 and PGF2α, found in high concentration at the inflammatory site [18]. From the preliminary invitro experiments and animal studies it is proved that, the active constituents of T.chebula are having immense pharmacological role in inhibiting many key constitutive and inducible enzymes involved in the pain pathways [19,20]. Shailasree et al., have indicated anti-inflammatory activity for the ethanolic extracts of fruits of T. chebula [21]. The compounds of T.chebula like Gallic acid (GA), ellagic acid (EA), and corilagin (CG) were reported to have anti-inflammatory activities by Phattarakom et al., [22]. Another study by Sang IKL et al., T. chebula is reported to contain hydrolysable tannins such as chebuline, chebulagic acid, gallic acid that have produced anti arthritic effect [23]. Moeslinger et al., demonstrated that the aqueous extract of dried fruit of T. chebula showed anti-inflammatory by inhibiting inducible nitric oxide synthesis [24].
In an in vitro study by Reddy DB et al., a dual inhibitory activity of chebulagic acid, extracted from Terminalia chebula on Cyclooxygenase-2 and 5-Lipo-Oxygenase. Additionally a down regulation of inflammatory mediator NFkB was also observed [14]. As NFkB is an important mediator in controlling multiple genes involved in inflammatory diseases like IBD, arthritis and gastritis. Thus down regulation of NFkB may be the possible underlying mechanism of Terminalia chebula [25]. Further T.chebula with its inherent pharmacological activity in preventing gastric ulcers could offer a new hope in using it concomitantly with NSAIDs and corticosteroids for chronic painful conditions like osteoarthritis and rheumatoid arthritis. In another study by Seo JB et al., reported anti-arthritic and analgesic effect of a standardized ethanol extract of T.chebula on collagen-induced arthritis and acetic acid-induced writhing model, respectively and the serum levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β were significantly reduced in mice [26]. Nair V et al., reported a significant inhibition of joint swelling by T.chebula in comparison to control in both formaldehyde induced and CFA-induced arthritis. It also reduced serum TNF-α level and synovial expression of TNF-R1, IL-6 and IL-1β. [27]. In evaluating the thermal pain in humans, many standard models like cold pressor, contact heat, radiant heat and laser have been used over time in research though associated with some limitations for each model [28].
This experimental data from animal studies prompted us to evaluate the analgesic effect of Terminalia chebula in hot air pain model in healthy human participants. To the best of our knowledge the present study tried to evaluate the pharmacodynamic activity of T.chebula in healthy human participants. For the evaluation of analgesic activity hot air pain model is chosen as it has been standardized, validated attaining a high degree of reproducibility with little variance by Sunil et al., [11]. The present experimental pain model utilizing tonic heat stimulation of the volar aspect of the forearm, to mimic clinical pain is simple and requires inexpensive equipment. In hot air pain model the main thermal pain carrying fibres are of Aδ and C type of fibres usually implicated in tonic stimulatory pain pathways. Similarly in another study, analgesic activity of Withania somnifera was evaluated using hot air analgesiometer with hot air pain model [12].
In our study, we observed a statistically significant increase in mean pain threshold, mean pain tolerance time, mean percentage change in pain threshold and tolerance with T.chebula 1000mg post drug when compared to baseline (pre drug).
Further Terminalia chebula capsules have demonstrated excellent safety profile in all the participants making them a good choice for using in chronic painful ailments especially in geriatric populations where predisposition for developing gastrointestinal and renal complications is most common.
Demographic characteristics of the study group
| Parameter | (n =12) |
| Age(years) | (33.18±4.24 |
| Weight (kg’s): | (64.10 ±6.3 |
| Height (cm’s): | (166.2 ±3.4 |
| BMI (kg/m2): | (23.2±1.031 |
Mean pain threshold and Mean pain tolerance for Placebo and T.chebula All Values presented as Mean ± SD
| Treatment group | Mean Pain threshold (in seconds) | Mean Pain tolerance (in seconds) |
|---|
| At baseline | Post drug 180minutes | At baseline | Post drug 180minutes |
|---|
| Placebo (n=12) | 34.11±2.78 | 34.59±2.62# | 49.05± 2.78 | 48.81±2.93# |
| T.chebula(n=12) | 34.06±2.63 | 41.00±2.99* | 49.67±3.72 | 57.3±3.21* |
*p<0.001 (compared to baseline and placebo)
#NS-Non Significant (compared to baseline)
Conclusion
In the present study, Terminalia chebula significantly increased pain threshold time and pain tolerance time compared to placebo. Study medications were well tolerated. Further studies may be needed to evaluate the analgesic effects in patients with chronic painful diseases like osteoarthritis and rheumatoid arthritis.
Acknowledgements
The authors thank ICMR Advanced centre for Clinical Pharmacodynamics, NIMS, Hyderbad for developing pain models, Natreon Inc for providing the proprietary extract of Terminalia chebula and placebo used in the present study and relevant literature and Dr. Sravanthi, Ayurvedic physician, for her expert advice.
*p<0.001 (compared to baseline and placebo) #NS-Non Significant (compared to baseline)
[1]. H Merskey, N Bogduk, art III: Pain Terms, A Current List with Definitions and Notes on Usage. Classification of Chronic Pain 1994 Second EditionIASP Task Force on Taxonomy:209-14. [Google Scholar]
[2]. C Guyton, JE Hall, Medical Physiology 2006 11th EditionChurchill Livingstone:598-609. [Google Scholar]
[3]. AH Zulfiker, R Mahbubur, H Kamal, K Hamid, ME Mazumder, MS Rana, In vivo analgesic activity of ethanolic extracts of two medicinal plants — Scoparia dulcis L. and Ficus racemosa linnBiol Med 2010 2:42-48. [Google Scholar]
[4]. E Hunsche, JV Chancellor, N Bruce, The burden of arthritis and nonsteroidal anti inflammatory treatment. A European literature reviewPharmacoeconomics 2001 19:1-15. [Google Scholar]
[5]. M Brennan, Adverse effects of NSAIDs on renal functionCanadian Medical Association Journal 1984 131(9):1012-13. [Google Scholar]
[6]. AM Trescot, S Datta, M Lee, H Hansen, Opioid pharmacologyPain Physician 2008 11(2):133-53. [Google Scholar]
[7]. K Bone, M Morgan, Clinical Applications of Ayurvedic and Chinese Herbs.Monographs for the Western Herbal PractitionerPhototherapy Press 1996 41:137-41. [Google Scholar]
[8]. MP Davis, Recent advances in the treatment of PainMedicine Reports 2010 2:63-772. [Google Scholar]
[9]. C Staahl, A Estrup, O Trin, A Lars, A Nielsen, AM Drewes, Human experimental pain models for assessing the therapeutic efficacy of analgesic drugsPharmacological reviews 2012 64(3):722-79. [Google Scholar]
[10]. Reddy KS kumar, Naidu MUR, Usha RP, Rao TR Kumar, Human experimental pain models: A review of standardized methods in drug developmentJ Res Med Sci 2012 17(6):587-95. [Google Scholar]
[11]. Reddy KS kumar, Naidu MUR, Usha RP, Rao TR Kumar, A simple thermal pain model for the evaluation of analgesic activity in healthy subjectsJ Anaesth Clin Pharmacol 2012 28(2):214-20. [Google Scholar]
[12]. Nalini P, Manjunath K, Kumar N, Reddy KS, Usharani P, Evaluation of the analgesic activity of standardized aqueous extract of Withania somnifera in healthy human volunteers using hot air pain modelResearch Journal of Life Sciences 2013 1(2):1-6. [Google Scholar]
[13]. JA Astin, Why patients use alternative medicine: Results of a national studyJournal of the American Medical Association 1998 279(19):1548-53. [Google Scholar]
[14]. DB Reddy, TC Reddy, G Jyotsna, S Sharan, N Priya, V Lakshmipathi, Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 celllineJ Ethnopharmacol 2009 30:506-12. [Google Scholar]
[15]. P Krishnamoorthy, S Vaithinathan, A Vimal Rani, A Bhuvaneswari, Effect of Terminalia chebula fruit extract on lipid peroxidation and antioxidative system of testis of albino ratsAfrican Journal of Biotechnology 2007 6(16):1888-91. [Google Scholar]
[16]. D Arome, AI Sunday, EI Onalike, A Amarachi, Pain and inflammation: Management by conventional and herbal therapyIndian Journal of Pain 2014 28(1):5-12. [Google Scholar]
[17]. S Sosa, MJ Balicet, R Arvigo, RG Esposito, C Pizza, G Altinier, Screening of the topical antiinflammatory activity of some central American plantsJ Ethanopharmacol 2002 8:211-15. [Google Scholar]
[18]. R Derardt, S Jongney, F Delvalcee, M Falhouta, Release of prostaglandins E and F in an algogenic reaction and its inhibitionEur J Pharmacol 1980 51:17-24. [Google Scholar]
[19]. S Kaur, RK Jaggi, Antinociceptive activity of chronic administration of different extracts of Terminalia bellerica Roxb. and Terminalia chebula Retz. fruitsIndian J Exp Biol 2010 48(9):925-30. [Google Scholar]
[20]. A Bag, S Kumar Bhattacharyya, N Kumar Pal, R Ranjan Chattopadhyay, Antiinflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract of Terminalia chebula fruitsPharm Biol 2013 51(12):1515-20. [Google Scholar]
[21]. S Sekhar, R Karmakar, KK Ramachandra, RN Siddapura, SP Harischandra, Potential anti-inflammatory bioactives from medicinal plants of Western Ghats, IndiaPharmacognosy Communications 2012 2(2):2-12. [Google Scholar]
[22]. P Rangsriwong, N Rangkadilok, J Satayavivad, M Goto, A Shotipruk, Subcritical water extraction of polyphenolic compounds from Terminalia chebula RetzSeparation and Purification Technology 2009 66:51-56. [Google Scholar]
[23]. IKL Sang, MH Pung, HK Seung, Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a natural product libraryArthritis Rheum 2005 52:345-53. [Google Scholar]
[24]. T Moeslinger, R Friedl, I Volf, M Brunner, E Koller, PG Spieckermann, Inhibition of inducible nitric oxide synthesis by the herbal preparation Padma 28 in macrophage cell lineCan J Physiol Pharmacol 2000 78(11):861-66. [Google Scholar]
[25]. C Monaco, E Andreakos, S Kiriakidis, C Mauri, C Bicknell, B Foxwell, Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosisPNAS 2004 101(15):5634-39. [Google Scholar]
[26]. JB Seo, SW Park, SS Choe, HW Jeong, JY Park, EW Choi, Anti-Arthritic and Analgesic Effect of NDI10218, a Standardized Extract of Terminalia chebula, on Arthritis and Pain ModelBiomol Ther. 2012 20(1):104-12. [Google Scholar]
[27]. V Nair, S Singh, YK Gupta, Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental modelsJ Pharm Pharmacol 2010 62(12):1801-06. [Google Scholar]
[28]. KSK Reddy, MUR Naidu, PU Rani, TRK Rao, Human experimental pain models: A review of standardized methods in drug developmentJ Res Med Sci 2012 17:587-95. [Google Scholar]