Introduction
Oro-pharyngeal cancer is more common in developing countries than the developed ones. Worldwide, oral cancer is one of the most prevalent cancers and is one of the 10 most common causes of death [1]. It has been established that virtually all oral cancers are preceded by visible clinical changes in the oral mucosa usually in the form of white or red patches termed as precancerous lesions and conditions [2].
However, in World Health Organization (WHO) Workshop, held in 2005, the term “potentially malignant” was preferred above “premalignant” or “precancerous”. Furthermore, it has been recommended to abandon the distinction between potentially malignant lesions and potentially malignant conditions and to use the term “potentially malignant disorders” (PMD) instead [3]. Of all the PMD, Leukoplakia, Oral submucus fibrosis, Oral lichen planus are the most common lesions.
Oral submucous fibrosis (OSMF) is a chronic, debilitating disease characterized by juxta epithelial fibrosis of the oral cavity. It is regarded as a precancerous and potentially malignant condition with incidence rates of 0.07–1.22% and malignant rates of 2.3–7.6%. Widely accepted definition of the disease as given by Pindborg and Sirsat (1966) is a chronic disease of insidious onset which can affect any part of the oral mucosa, rarely oropharynx .The disease starts initially with formation of vesicles with stomatitis, leading to fibro-elastic changes in the lamina propria causing fibrosis that leads to trismus, epithelial atrophy leading to burning sensation and inability to eat [4].
Hyperbaric Oxygen Therapy (HBOT)
Hyperbaric Medicine is the clinical specialty using pressure higher than local atmospheric pressure (>1atm) to treat diseases or injuries inside a hyperbaric chamber, to derive therapeutic benefit from breathing gases, usually O2. Hyperbaric Oxygen Therapy—“HYPER” means increased and “BARIC” means pressure.
The concept of using respiratory gases at ambient pressures in the treatment of illnesses dates back three centuries. In 1662 hyperbaric air was used by Henshaw for the treatment of “affections of the lung” [5]. In 1834 Junod (France), built a chamber to treat pulmonary conditions at pressures between 2 and 4 atmospheres absolute (ATA). Hyperbaric air was used to treat a wide variety of ailments, including cardio – pulmonary disease, carcinomas, diabetic foots and psychological disorders and was used as an aid to surgery, providing “deeper anaesthesia and less cyanosis”. Orval J Cunningham in the early 1900s, successfully treated sufferers of the Spanish flu epidemic with hyperbaric air.
Definition
The Committee on Hyperbaric Medicine defines HBOT therapy as “A mode of medical treatment in which the patient is entirely enclosed in a pressure chamber and breathes 100% oxygen at a pressure >1 atmosphere absolute (ATA).” ATA is the unit of pressure and 1 ATA is equal to 760 mm of mercury or pressure at sea level.
Mechanisms of action
The clinical benefits of hyperbaric oxygen can be explained by the
Mechanical effects of pressure,
Physics of gas laws ,
The physiological and biochemical effects of hyperoxia and
Through the reversal of local hypoxia in target tissues [6,7].
When we normally breathe air (with 21% O2) at sea level pressure, most tissues need of oxygen are met from the oxygen combined to hemoglobin, which is 95% saturated. 100 ml blood carries 19 ml O2 combined with hemoglobin and 0.32 ml dissolved in plasma. At the same pressure if 100% O2 (oxygen) is inspired, O2 combined with hemoglobin increases to a maximum of 20 ml and that dissolved in plasma to 2.09 ml [Table/Fig-1].
Multiplane Hyperbaric chamber

HBOT causes increase in the diffusion of more oxygen under raised atmospheric pressure into solution i.e., plasma component of the blood. The amount of O2 dissolved in plasma raises from 4.4 ml/dL to 6.8 ml/dL with increase in 1 ATA. This raised O2 levels in plasma are responsible to meet oxygen demand in hypoxic areas irrespective of blood hemoglobin levels and amount of Oxyhemoglobin, this forms the rationale of this therapy in treating certain hypoxic conditions such as Carbon monoxide poisoning, etc and hemoglobin pathologies such as anaemias, cyanosis, etc.
Few Physiological and biochemical effects of hyperoxia [
8]
Improved leucocyte killing activity.
Promotion of angioneogenesis in problem wounds, flaps and irradiated tissues.
Reduced falls in adenosine triphosphate (ATP) and phosphocreatinine levels in burns.
Decreased white cell adherence to capillary walls.
Vasoconstriction in normal blood vessels.
Decreased post-traumatic tissue oedema.
Decreased lipid peroxidation.
Few Indications for HBOT Approved by the Undersea and Hyperbaric Medical Society [
9]
Decompression sickness and Air or gas embolism.
Carbon monoxide poisoning .
Clostridial myositis and myonecrosis (gas gangrene).
Intracranial abscess, actinomycosis.
Necrotising soft tissue infections.
Skin grafts and flaps (compromised).
Contraindications for HBOT [
8]
Absolute
Untreated tension pneumothorax.
Relative
Upper respiratory tract infection.
Asymptomatic pulmonary lesions seen on chest x-ray.
History of thoracic or ear surgery.
Pregnancy.
Claustrophobia.
Middle ear barotrauma is the most common complication of HBOT therapy, with an incidence of about 2%. Inner ear barotrauma is a very rare occurrence [10].
Sinus squeeze is the second most common complication of HBOT therapy.
Tooth pain can occur during compression or decompression and this typically follows dental treatment that has created an air space under a dental filling.
Pulmonary oxygen toxicity can occur in patients if exposed for prolonged periods.
Fire is the most common fatal complication of hyperbaric oxygen therapy.
Over the last 20 years, 52 deaths have been reported, most due to inadequate fire precautions [11].
Indications of HBOT in Dentistry [
12]
Osteoradionecrosis
Postradiotherapy cases
Mandibular Osteomyelitis, Chronic Refractory Osteomyelitis
Periodontal disease
Infected Implants
Effects of Hbotin Osmf – A Molecular Level Approach
1. Collagen Metabolism
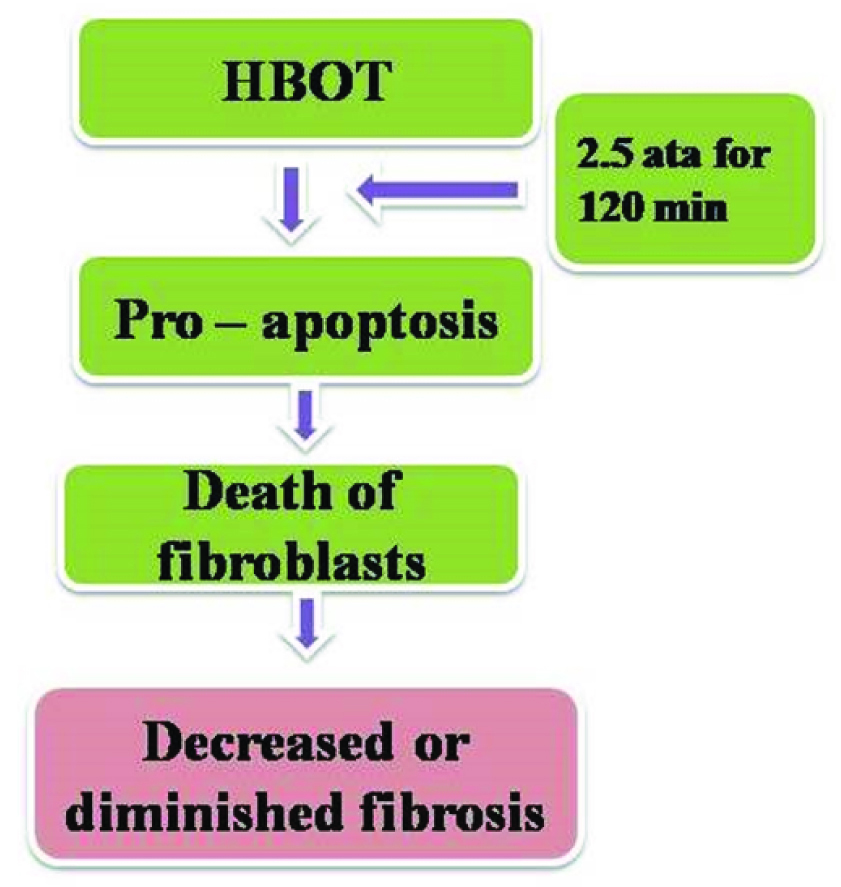
In OSF increased fibrosis is due to imbalance between activation of fibroblasts and reduced degradation of collagen leading to increased fibrosis and trismus. Conconi et al., found that exposure to HBOT at 2.5 ATA for 120 min enhanced apoptosis of mouse fibroblast cell line [13]. HBOT also reduced cell proliferation and promoted cell death when skin fibroblasts were cultured in a high-glucose medium at 2.5 ATA for 90 min on three consecutive days [14].
HBOT may induce apoptosis of lymphocytes or/and decrease lymphocytic proliferation so as to keep fibroblasts from activating cytokines. HBOT at 1.0 or 2.5 ATA for 30 and 60 min enhanced 3T3/J2 fibroblast cell growth while at 2.5 ATA for 120 min, it exerted a pro apoptotic effect. HBOT may potentially contribute to the inhibition of fibroblasts by reducing IL-1b and TNF-α production [13,15,16]. HBOT may be useful in the treatment for OSF by promoting fibroblast apoptosis and inhibiting fibroblast activation [17] [Table/Fig-2].
Image showing effect of HBOT on fibroblasts
2. Down Regulation of TGF-β and IFN-γ
TGF-β expression, IFN secretion and the growth of fibroblasts decreased after chronic exposure to HBOT [18].
3. Suppression of TNF-α Secretion
TNF-α could up-regulate mRNA expression of collagen types I and III in cultured lung fibroblasts [19]. This suggests that HBO may have the potential to treat OSF by inhibiting TNF-α and influencing the synthesis of collagenase [17] [Table/Fig-3].
Image showing effect of HBOT on inflammatory mediators

Oxidative stress occurs when the formation of oxygen free radicals exceeds the antioxidant defense capabilities [20]. It is established that the lipid peroxidation increases with severity of the disease which is reflected in increase in the plasma malondialdehyde levels (marker of oxidative stress and lipid peroxidation) when compared to healthy controls.
HBOT treatment provides extra oxygenation of the tissues of the whole body, decrease in the production of reactive oxygen species, lipid peroxidation and increasing the antioxidant activity of enzymes such as Superoxide dismutase, glutathione peroxidase, catalase, paraoxonase, and heme-oxygenase-1 [21,22].
5. Inflammation
Continuous contact between the quid and oral mucosa resulting in absorption and metabolism of the alkaloids in the quid resulting in the chronic inflammation causing activation of macrophages and T cells and an increase in the level of cytokines such as IL6, TNF, IF-α and TGF- β [23].
HBOT has potent anti-inflammatory tissue effects. It has been shown to attenuate the production of pro-inflammatory cytokines including TNF-α, IL-1, IL-1b, and IL-6 and increase the production of anti-inflammatory IL-10 [15,24].
6. Hypoxia
Extensive fibrosis of the connective tissue causes reduction of vascularity, resulting in subsequent hypoxia in both fibroblasts and surface epithelia. Hypoxia causes atrophy and ulceration of the epithelium by inducing apoptosis. In addition, the over expression of hypoxia-induced factor-1a is seen in OSMF, which indicates changes in cell proliferation, maturation, and metabolic adaptation increasing the possibility of malignant transformation [25].
OSMF is now considered as a collagen metabolism disorder, OSMF fibroblasts show 1.5 times increased activity of collagen production, when compared to their healthy counterparts. However, as the disease progresses Type III collagen is almost completely replaced by Type I collagen, and subsequent collagen is considered to be more resistant to regular collagen degradation process [25]. Increased fibrosis leads to relative ischemia and subsequent hypoxia of superficial layers causing epithelial atrophy and ulceration suggesting over expression of Hypoxia-induced factor-1a (HIF-1a). Hypoxia-induced factor-1a plays key role in explaining the possibility of malignant transformation in OSMF [26].
HIF-1a was up-regulated at both the protein and mRNA levels in OSF, which suggested that it may contribute to the progression of fibrosis. Early stages of OSF showed increased vascular density and obviously decreased in the middle and late stages, blocking capillary angiogenesis [17].
HBOT increases oxygen tension, enhances the amount of dissolved oxygen in the plasma, and raises oxygen delivery to the hypoxic areas. HBOT improved ischemia via decreasing expression of HIF-1a [27]. The anti-inflammatory effect of HBOT might occur through the relief of hypoxia and the down-regulation of HIF-1a [28]. HBOT may have the potential to improve the vascular situation.
Role of micro nutrients
Copper is present in high quantities of areca-nut causing it to increase in the blood picture of a chronic gutkha chewer (OSF ) The enzyme lysyl oxidase is found to be upregulated in OSF [29] which is a copper dependent enzyme and plays a key role in collagen synthesis and its cross linkage making it resistant to collagen degradation. HBOT also increases zinc, decreases copper, and increases ceruloplasmin levels [30].
Conclusion
OSMF is having highest malignant potential than any other oral potentially malignant disorders, in which etio-pathogenesis is poorly understood despite the recent advances. Novel treatment modality such as HBOT in the management of OSMF has been studied. HBOT not only has a cellular-regulation effect, but also plays a role in the management of various cytokines and transcription factors for angiogenesis and anti-inflammatory at cellular and molecular level .
HBOT may be considered as a potential supplementary therapy to improve the localized hypoxic microenvironment of pre cancers such as OSMF, Erosive lichen planus etc. Therefore, more evidence-based, randomized, and controlled studies need to be conducted with HBOT on larger samples to find out the most suitable doses and efficacy of it in treating OSMF.
[1]. Petersen PE, Guest Editorial. Strengthening the prevention of oral cancer: The WHO perspectiveComm Dent Oral Epidemiol 2005 33:397-99. [Google Scholar]
[2]. Reibel J, Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristicsCrit Rev Oral Biol Med 2003 14:47-62. [Google Scholar]
[3]. Warnakulasuriya S, Johnson NW, van der Waal I, Nomenclature and classification of potentially malignant disorders of the oral mucosaJ Oral Pathol Med 2007 36:575-80. [Google Scholar]
[4]. Pindborg JJ, Sirsat SM, Oral submucous fibrosisOral Surg Oral Med Oral Pathol 1966 22:764-79. [Google Scholar]
[5]. Simpson A, Compressed air as a therapeutic agent in the treatment of consumption, asthma, chronic bronchitis and other diseases 1857 EdinburghSutherland and Knox:1-4. [Google Scholar]
[6]. Boerema I, Meyne NG, Brummel Kamp WK, Life without blood: A study of the influence of high atmospheric pressure and hypothermia on dilution of bloodJ Cardiovasc Surg 1960 1:133-46. [Google Scholar]
[7]. Lambertsen CJ, Kough RH, Cooper Dy, Oxygen toxicity: effects in man of oxygen inhalation at 1 and 3.5 atmospheres upon blood gas transport, cerebral circulation and cerebral metabolismJ Appl Physiol 1953 5:471-86. [Google Scholar]
[8]. Sharkey S, Current indications for hyperbaric oxygen therapyAdf health 2000 1:64-72. [Google Scholar]
[9]. Undersea and hyperbaric medical society. Hyperbaric oxygen therapy: a committee report. Maryland, 1996 1; 15-18 [Google Scholar]
[10]. Davis JC, Dunn JM, Heimbach RD, Hyperbaric medicine: patient selection, treatment procedures, and side effectsElsevier science 1988 :233-235. [Google Scholar]
[11]. Sheffield PJ, Desautels DA, Hyperbaric and hypobaric chamber fires: a 73 year analysisUndersea hyperbar med 1997 24:153-64. [Google Scholar]
[12]. Jain N, Deepa D, Applications of hyperbaric oxygen therapy in dentistry: A mini reviewJournal of Interdisciplinary Dentistry 2014 4(1):27-32. [Google Scholar]
[13]. Conconi MT, Baiguera S, Guidolin D, Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3t3/j2 cell lineJ Investig Med 2003 51(4):227-32. [Google Scholar]
[14]. Lin hi, Chu SJ, Perng WC, Wu CP, Hyperbaric oxygen attenuates cell growth in skin fibroblasts cultured in a high-glucose mediumWound Repair Regen 2008 16(4):513-19. [Google Scholar]
[15]. Benson RM, Minter LM, Osborne BA, Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophagesClin Exp Immunol 2003 134(1):57-62. [Google Scholar]
[16]. Gadd MA, Mcclellan DS, Neuman TS, Effect of hyperbaric oxygen on murine neutrophil and t-lymphocyte functionsCrit Care Med 1990 18(9):974-79. [Google Scholar]
[17]. Ye X, Zhang J, Lu R, HBO: A possible supplementary therapy for oral potentially malignant disordersMedical Hypotheses 2014 83(2):131-36. [Google Scholar]
[18]. Hopf HW, Gibson JJ, Angeles AP, Constant Hyperoxia and angiogenesisWound Repair Regen 2005 13(6):558-64. [Google Scholar]
[19]. Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN, Enhanced il-1 beta and tumor necrosis factor-alpha release and messenger rna expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposureJ Immunol 1993 150(9):4188-96. [Google Scholar]
[20]. Rossignol DA, Hyperbaric oxygen therapy might improve certain pathophysiological findings in autismMedical Hypotheses 2007 68:1208-27. [Google Scholar]
[21]. Cuzzocrea S, Imperatore F, Costantino G, Role of hyperbaric oxygen exposure in reduction of lipid peroxidation and in multiple organ failure induced by zymosan administration in the ratShock 2000 13(3):197-203. [Google Scholar]
[22]. Ozder TA, Uzun H, Bohloli M, The effects of hyperbaric oxygen treatment on oxidant and antioxidants levels during liver regeneration in ratsTohoku J Exp Med 2004 203(4):253-65. [Google Scholar]
[23]. Dyavanagoudar SN, Oral submucous fibrosis: review on etiopathogenesisJ Cancer Sci ther 2009 1:72-77. [Google Scholar]
[24]. Weisz G, Lavy A, Adir Y, Modification of in vivo and in vitro tnf-alpha, il-1, and il-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal crohn’s diseaseJ Clin Immunol 1997 17(2):154-59. [Google Scholar]
[25]. Utsunomiya H, Tilakaratne WM, Oshiro K, Maruyama S, Suzuki M, Ida-Yonemochi M, Extracellular matrix remodeling in oral submucous fibrosis; its stage-specific modes revealed by immuno-histochemistry and in-situ hybridizationJ Oral Pathol Med 2005 34:498-507. [Google Scholar]
[26]. Tilakaratne WM, Iqbal Z, Teh MT A, Upregulation of HIF-1a in malignant transformation of oral submucous fibrosisJ Oral Pathol Med 2008 37:372-77. [Google Scholar]
[27]. Aubert B, Bona M, Boutigny D, Observation of tree-level b decays with ss production from gluon radiationPhys Rev Lett 2008 100(17):171803.s [Google Scholar]
[28]. Thackham JA, Mcelwain DLS, Long RJ, The use of hyperbaric oxygen therapy to treat chronic wounds: a reviewWound repair regen 2008 16(3):321-30. [Google Scholar]
[29]. Trivedy CR, Warnakulasuriya Kaas, Hazarey VK, The upregulation of lysyl oxidase in oral submucous fibrosis and squamous cell carcinomaJ Oral Med Pathol 1999 28:246-51. [Google Scholar]
[30]. Marx RE, Ehler WJ, Tayapongsak P, Relationship of oxygen dose to angiogenesis induction in irradiated tissueAm J surg 1990 160(5):519-24. [Google Scholar]