A Study on Longevity of Immune Response after Vaccination with Salmonella Typhi Vi Conjugate Vaccine (Pedatyph™) in Children
Balaji Chinnasami1, Kanimozhi Sadasivam2, Aravindhan Vivekanandhan3, Prema Arunachalam4, Sekar Pasupathy5
1Associate Professor, Department of Paediatrics, SRM MCH& RC, Chennai, India.
2Assistant Professor, Department of Physiology, SRM MCH& RC, Chennai, India.
3Researcher, Department of Immunology and Molecular Biology, AU-KBC Research Centre, MIT Campus of Anna University, Chrompet, Chennai, India.
4Former, Professor & HOD, Department of Paediatrics, SRM MCH& RC, Chennai, India.
5Professor & HOD, Department of Paediatrics, SRM MCH& RC, Chennai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Balaji Chinnasami, Associate Professor, Department of Paediatrics, SRM Medical College Hospital & Research Center, Kattankulathur, Chennai -603203, India.
E-mail: balajictriumphants@gmail.com
Background and Objectives
Owing to the limitations of the conventional polysaccharide vaccines, global efforts have been made to develop conjugated polysaccharide vaccines for typhoid. Duration of immune response induced by these vaccines is critical to define the efficacy and frequency of required booster dose.
This study was done to determine the duration of immune response following vaccination with Salmonella Typhi Vi conjugate vaccine (Pedatyph™) in children and to assess the booster effect of second dose of conjugate typhoid vaccine.
Materials and Methods
Forty children were recalled from a cohort of 400 children, who received one dose or two doses of PedaTyph™, 30 months after vaccination. Ten non-vaccinated children were also recalled. Their serum samples were assessed by ELISA for anti Vi antibody.
Results
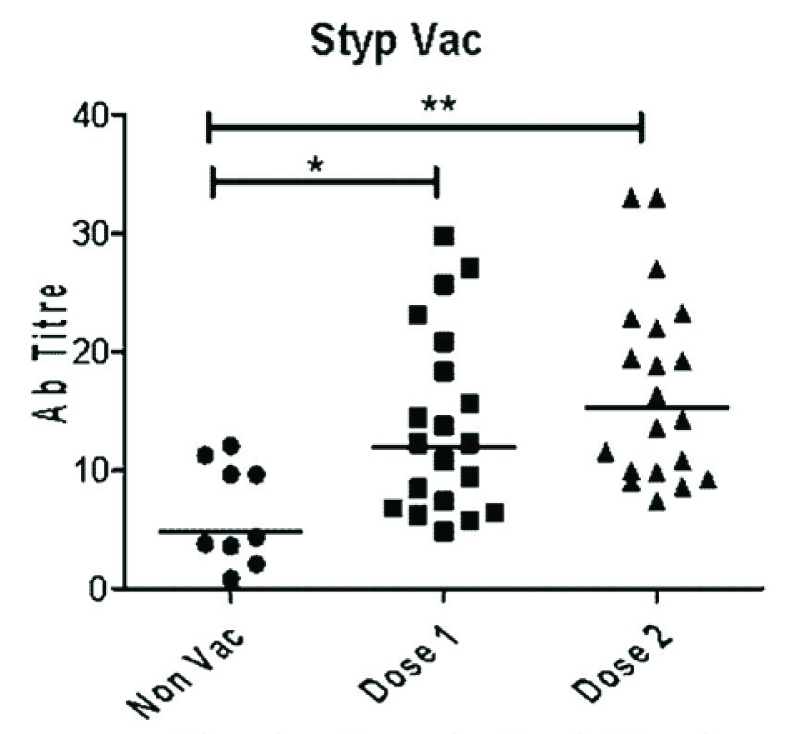
Significantly high titers of anti-Vi polysaccharide IgG antibodies were present in vaccinated children even after 30 months of vaccination as compared to non-vaccinated children. Geometric mean titers (GMT) with 95% confidence intervals were 14 (4.8-29.8), 17 (7.4-33) and 6.4 (0.8-12) μg/ml for single dose, two doses and control group respectively. The children in two doses group had higher antibody titers as compared to single dose group. However, the difference was not significant.
Interpretation and Conclusion
PedaTyph™ was found to induce long term immune response as evidenced by presence of significant anti-Vi polysaccharide antibodies after 30 months of vaccination. No significant advantage of two doses regimen over one dose was found. Hence one dose vaccination with PedaTyph™ is suggested.
Salmonella, Salmonella Typhi, Typhoid, Vaccine, Conjugate typhoid vaccine
Introduction
Typhoid is a serious childhood disease especially in developing countries. Globally approx 21 million children are affected annually [1]. In Asia, rates of blood culture confirmed typhoid fever among children of five to fifteen years of age ranged from 180 to 494 cases per 100,000 children [2]. Typhoid is generally believed to be a disease of older children but studies have indicated that typhoid causes significant morbidity and mortality in children below five years of age [3-5]. Vaccination is recommended as a key intervention in the control of typhoid fever [6] especially given the emergence of multidrug resistant strains of S. Typhi [7,8].
A simple vaccine based on capsular polysaccharide Vi of S. Typhi has proven moderately efficacious in typhoid endemic areas [9,10]. However, a polysaccharide alone vaccine has its limitations as it is not suitable for immunization of infants and young children because of its T cell independent properties [11]. These limitations have been overcome to a great extent by conjugation of the Vi polysaccharide to protein moieties [12]. The experimental conjugated typhoid vaccine developed at the US National Institutes of Health (NIH) was found to be safe and effective in field trials carried out in Vietnam [13,14].
The first commercially available Vi antigen conjugated with tetanus toxoid is manufactured by Bio-Med (P) Ltd in India and is available by trade name Peda Typh™ since licensure in 2008. Serological immune response was demonstrated in 100% of vaccinated infants and young children after three weeks of vaccination in a clinical trial sponsored by the company and published on their website [15]. Considering the need for further studies, we conducted a safety and immunogenicity trial in 2011 using Peda Typh™ [16]. In that study, 400 children were randomized to receive either one dose (Group A) or two doses (Group B) of Vi conjugate typhoid vaccine.
Pasupathy5We demonstrated that a single dose of conjugated typhoid vaccine (Pedatyph™) resulted in seroconversion in 83% of children and mean antibody titers rose by nine fold at eight weeks post vaccination.
The present studies were conducted to assess the long term immune response after vaccination with Peda Typh™ as this has a bearing on duration of protection provided by Peda Typh™.
Materials and Methods
Vaccine
The conjugate typhoid vaccine used, Peda Typh™, was received as donation from Bio-Med (P) Ltd, Ghaziabad, India. The vaccine was supplied by the manufacturer from the regular commercial stocks in lots of 50 vials at a time as requested from time to time during the study period. Each dose of the vaccine contains 5µg of Vi polysaccharide of S. Typhi conjugated to 5µg of tetanus toxoid. The vaccine was stored at 2-8ºC in a refrigerator as per manufacturer’s recommendations until used.
Study Design
In a previous randomized comparative clinical trial, 400 children with age <5 y were vaccinated either one dose (group A) or two doses (group B) of the vaccine [16]. We recalled 40 vaccinated children, who were earlier vaccinated with conjugate typhoid vaccine, 30 months later. Antibody titers of unconjugate typhoid vaccine usually wane by 30-36 months, necessitating booster doses. Hence, we chose 30 months as post vaccination follow-up period. Twenty children were from Group A (Single dose) and remaining twenty children were from Group B (two doses eight weeks apart). We also included ten children (age and gender matched) not vaccinated with typhoid vaccine as control group. The study was approved by Ethical committee of SRM medical college and follow up study protocol was incorporated in Clinical Trials Registry of India (CTRI/2010/091/003031). Informed written consent was obtained from parents of all children recruited in the follow up study. This cross sectional study was conducted in the Department of Paediatrics, SRM Medical College from May 2014 to August 2014. Two ml of blood was collected from all children, serum separated and stored at -20 degree Celsius. All collected samples were transported to AU-KBC research centre, MIT campus of Anna University where analyses of samples was done.
Serological Testing
Vi antibody titers were measured by ELISA that was a modification of ELISA technique of the NIH, U.S.A [17]. Human IgG anti-Vi reference sera for S. Typhi with strength of 33 μg/ml was kindly donated by Dr. Shousun C. Szu of theNIH, USA [18]. All the remaining reagents for ELISA were provided by Bio-Med (P) Ltd. ELISA was carried out as described briefly below.
Wells of a microtitre plate were coated with 0.4 mg/well of Vi polysaccharide antigen covalently bound to bovine serum albumin. Dilutions of serum to be tested (1:100 for pre-immune serum and 1:100, 1:400 for post-immune serum) were made and added to the wells. NIH standard reference anti Vi polysaccharide human serum was used as reference. Anti human IgG-HRP conjugate and substrate for peroxidase were added sequentially. The optical density was measured in ELISA reader at 492 nm wavelength. A standard curve was generated using a four parameter fit. Antibody titers in test sera were calculated from this curve.
Results
Baseline characteristics were comparable among all three groups. The mean age was 4.5 y and 58% were boys. The median follow up period for previously vaccinated children was 30 months. Geometric mean titers (GMT) with 95% confidence intervals for single dose, two doses and control group were 14(4.8-29.8), 17(7.4-33) and 6.4 (0.8-12) μg/ml respectively. Low levels of anti-Vi polysaccharide IgG antibodies could be detected even in non-vaccinated subjects. However, as can be seen in [Table/Fig-1] vaccinated subjects had significantly high titers of anti-Vi polysaccharide IgG antibodies compared to non-vaccinated subjects, even after 30 months of vaccination. Even though GMT in two dose group was higher than single dose group the difference was not significant.
Discussion
The Vi capsular polysaccharide (Vi antigen) of S. Typhi is well-recognized as the necessary factor for virulence and as antigen that confers immunity against the typhoid fever [9]. However, like any other polysaccharide, Vi antigen alone does not induce immunity in infants below two years of age due to its ‘T” cell independent nature. Thus, it is unsuitable for protection of infants and young children [11].
Vi antigen has been conjugated with various protein carriers eg rEPA (recombinant exoprotein A of Pseudomonas aeruginosa [17], di-O-acetyl pectin protein [19], cholera toxin [20], B subunit of the heat labile toxin of E. coli [21] or diphtheria toxoid and tetanus toxoid [22] to produce experimental vaccines. Bio-Med (P) Ltd uses tetanus toxoid as protein carrier to prepare conjugated Vi antigen vaccine that is licensed and marketed as Peda Typh™ in India since 2008 [23].
Peda Typh™ was the first conjugated Vi antigen vaccine tested on infants of 3 months and older age. The vaccine was found safe, least reactogenic and induced immune response (>4 fold) in 100% of vaccinees tested after three weeks of vaccination [15]. Clinical trials conducted in Vietnam Mekong Delta area using Vi-rEPA conjugated vaccine experimentally produced by NIH, USA demonstrated 89% efficacy over a trial period of 46 months in 2-5-year-old children [14]. Even though rEPA conjugated Vi antigen has been tested in clinical trials for protective efficacy and serological responses for approximately four years, it was considered desirable to test the immunological response of Vi antigen conjugated to tetanus toxoid (Peda Typh™) vaccine in infants and young children over a 30 month period and record protective efficacy of the vaccine in Indian conditions.
In our previous study we assessed the safety and immunogenicity of Peda Typh™ vaccine [16]. Adverse events occurred in 17% of children after first dose and 21% of children after second dose. But adverse events were not serious and children recovered within 48 h. We demonstrated 83% seroconversion and increase in mean antibody titers by 9 fold. We conducted this follow up study to assess long term persistence of immunogenicity and to assess the booster effect of second dose. From the present study it is interesting to note that vaccinated children had significantly high titers of anti-Vi antibodies even after two and half years of vaccination compared to non-vaccinated children. Seven children in single dose group (Group A) and ten children in two doses group (Group B) had titers above 15ug/ml whereas no child in control group had such high titers. These findings clearly suggest that antibodies generated after vaccination with Peda Typh™ persist at 30 months after vaccination.
The antibody titers in two doses group were higher than single dose group but the difference was not statistically significant. This finding suggests that there is no significant booster effect by second dose of conjugate typhoid vaccine. Phase 2 clinical trial results of other conjugate typhoid vaccines (Vi-CRM197 & Typbar-TCV) also show that there is no incremental effect on antibody titers due to second dose [24]. Our findings support IAP COI recommendation of giving one dose of conjugate typhoid vaccine [25].
Serum antibody titres in Peda Typhvaccinated and non-vaccinated children. The geometric mean is represented by the horizontal bars p values were calculated by Kruskal–Wallis one-way analysis of variance p 0.05 was considered significant
*p<0.05 Dose 1 compared to Non Vac group
**p<0.05 Dose 2 compared to Non Vac group
Limitations
However, our study has few limitations. Large field effectiveness trial would be preferable to a serological study like the present one. It is desirable to compare with other commercially available conjugate and unconjugate vaccine brands. A further five year follow-up study is needed.
Conclusion
From our study we can conclude that Peda Typh™ is safe and immunogenic even after 30 months of vaccination. Second dose of Peda Typh™ given after eight weeks of first dose didn’t produce any booster effect and hence single dose is recommended.
Acknowledgement
Special thanks to Dr. Shousun C. Szu from NIH, USA for providing the Reference Standards for analysis.
Conflict of Interest: The vaccines were provided by Bio-Med (P) Ltd, Ghaziabad, India. But Bio-Med had no role in planning the study design, data analysis and manuscript writing.
[1]. JA Crump, SP Luby, ED Mintz, The global burden of typhoid fever. BullWorld Health Organ 2004 82:346-53. [Google Scholar]
[2]. RL Ochiai, CJ Acosta, MC Danovaro-Holliday, Domi Typhoid Study Group. A study of typhoid fever in five Asian countries: disease burden and implications for controlsBull. World Health Organ 2008 86(4):260-68. [Google Scholar]
[3]. MO Stormon, PB McIntyre, J Morris, B Fasher, Typhoid fever in children: Diagnostic and therapeutic difficultiesPediatric Infect Dis J 1997 16:713 [Google Scholar]
[4]. A Sinha, S Sazawal, R Kumar, S Sood, VP Reddaiah, B Singh, Typhoid fever in children aged less than 5 yearsLancet 1999 354(9180):734-37. [Google Scholar]
[5]. M Verma, Y Parashar, A Singh, R Kamoji, Current pattern of enteric fever: a prospective clinical and microbiological studyJ Indian Med Assoc 2007 105:582-586. [Google Scholar]
[6]. D DeRoeck, RL Ochiai, J Yang, DD Anh, V Alag, JD Clemens, Typhoid vaccination: the Asian experienceExpert Rev Vaccines 2008 7(5):547-60. [Google Scholar]
[7]. AC Anand, VK Kataria, W Singh, SK Chatterjee, Epidemic multiresistant enteric fever in eastern IndiaLancet 1990 335:52 [Google Scholar]
[8]. J Wain, C Kidgella, The emergence of multidrug resistance to antimicrobial agents for the treatment of typhoid feverTrans R Soc Trop Med Hyg 2004 98:423-30. [Google Scholar]
[9]. JD Robbins, JB Robbins, Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella TyphiJ Infect Dis 1984 150:436-49. [Google Scholar]
[10]. IL Acharya, CU Lowe, R Thapa, VL Gurubacharya, MB Shrestha, M Cadoz, Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella TyphiA preliminary report. N Engl J Med 1987 317(18):1101-04. [Google Scholar]
[11]. M Cadozn, Potential and limitations of polysaccharide vaccines in infancyVaccine 1998 16(14-15):1391-95. [Google Scholar]
[12]. A Podda, A Saul, R Arora, Z Bhutta, A Sinha, Conjugate vaccines for enteric fever: Proceedings of a meeting organized in New Delhi, India in 2009J Infect Dev Ctries 2010 4:404-11. [Google Scholar]
[13]. FY Lin, VA Ho, HB Khiem, DD Trach, PV Bay, The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old childrenN Engl J Med 2001 344:1263-69. [Google Scholar]
[14]. NL Mai, VB Phan, AH Vo, CT Tran, FY Lin, Persistent efficacy of Vi conjugate vaccine against typhoid fever in young childrenN Engl J Med 2003 349:1390-91. [Google Scholar]
[15]. SP Goel, PS Reddy, MP Singh, Clinical trial of Vi conjugate typhoid vaccine (PedaTyph™)http://biomed.co.in/typh_trial.html Accessed January,2015 [Google Scholar]
[16]. B Chinnasami, V Mangayarkarasi, A Prema, K Sadasivam, MJ Davis, Safety and Immunogenicity of Salmonella Typhi Vi conjugate vaccine (PedaTyph™) in children upto five yearsInternational Journal of Scientific and Research Publications. IJSRP 2013 3(2):1-5. [Google Scholar]
[17]. Z Kossaczka, FY Lin, VA Ho, NT Thuy, P Van Bay, TC Thanh, Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers,and 2- to 4-year-old children in VietnamInfect Immun 1999 67(11):5806-10. [Google Scholar]
[18]. SC Szu, S Hunt, G Xie, A human IgG anti-Vi reference for Salmonella Typhi with weight-based antibody units assignedVaccine 2013 1(15):1970-74. [Google Scholar]
[19]. Z Kossaczka, S Bystricky, DA Bryla, J Shiloach, JB Robbins, SC Szu, Synthesis and immunological properties of Vi and di-O-acetyl pectin protein conjugates with adipic acid dihydrazide as the linkerInfect Immun 1997 65(6):2088-93. [Google Scholar]
[20]. SC Szu, XR Li, R Schneerson, JH Vickers, D Bryla, JB Robbins, Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight ViInfect Immun 1989 57(12):3823-27. [Google Scholar]
[21]. SC Szu, DN Taylor, AC Trofa, JD Clements, J Shiloach, JC Sadoff, Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccinesInfect Immun 1994 62(10):4440-44. [Google Scholar]
[22]. SC Szu, AL Stone, JD Robbins, R Schneerson, JB Robbins, Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animalsJ Exp Med 1987 166(5):1510-24. [Google Scholar]
[23]. T Singhal, Y Amdekar, R Agarwal, IAP guidelines on immunization: I.A.P. Committee on Immunization. 2007-2008 2009 New DelhiJaypee:57 [Google Scholar]
[24]. ZA Bhutta, Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: Results from two randomised, observer-blind, age de-escalation, phase 2 trialsLancet Infect Dis 2014 14(2):119-29. [Google Scholar]
[25]. M Vipin, VP Choudhury, Kalra A, Indian Academy of Pediatrics (IAP) Recommended Immunization Schedule for Children Aged 0 through 18 years – India, 2014 and Updates on ImmunizationIndian Pediatrics 2014 51:785-803. [Google Scholar]