Multiple myeloma is a cancer of plasma cells. It is characterized by a clonal proliferation of malignant plasma cells in bone marrow, monoclonal protein, osteolytic bone lesions, renal failure and immunodeficiency [1]. The cells may cause soft-tissue masses (plasmacytomas) or lytic lesions in the skeleton. The presentation of multiple myeloma can range from being asymptomatic to severely symptomatic with complications requiring immediate treatment. The clinical manifestations are anemia, leucopenia, renal failure, pathologic fractures, spinal cord compression, recurrent infections, peripheral neuropathy, hyperviscosity, etc [2].
Multiple myeloma accounts for 1% of all neoplastic disorders and 10% of all hematological malignancies. A study in India has shown that the incidence of multiple myeloma in the year 2005 varied from 0.3 to 1.9 per 100,000 for men and 0.4 to 1.3 per 100,000 for women [3]. Its incidence in North America is 4.8 per 100,000 population for men and 3.3 per 100,000 for women [1].
A combination of melphalan and prednisone was first used to treat multiple myeloma in 1960s, and median patient survival increased to 2 to 3 y. This combination remained a mainstay of myeloma therapy for decades [4,5]. High-dose melphalan followed by bone marrow (BM) transplantation and peripheral blood stem cell grafting increased median survival to 3 to 4 y [1,2]. An important problem of melphalan therapy was that it leads to late and long-lasting myelosuppression and increased risk of developing acute myeloid leukaemia. Hence,in patients who were eligible for stem cell transplantation a combination of vincristine, doxorubicin and dexamethasone (VAD) was used for many years as induction therapy. A combination of melphalan and prednisone was used as induction therapy in patients who were ineligible for stem cell transplantation [3–5].
There have been major advances in the treatment of multiple myeloma in the last decade. Drugs like thalidomide, lenalidomide and bortezomib have emerged as active drugs in the treatment of multiple myeloma. The most common induction regimens used presently are thalidomide–dexamethasone, lenalidomide– dexamethasone and bortezomib based regimens. Studies demonstrated that combination of each of these agents with dexamethasone produced superior response in newly diagnosed multiple myeloma patients as compared to dexamethasone alone and other existing combination regimens [6]. Lenalidomide plus Dexamethasone is effective for relapsed or refractory multiple myeloma cases [7]. Thus, combinations of these new drugs with dexamethasone are currently used as initial therapy in patients eligible for transplantation.These advances in therapy have helped to decrease the occurrence and severity of complications of multiple myeloma [5,8]. Bortezomib, has been shown to be effective in patients with relapsed multiple myeloma that is refractory to conventional chemotherapy [9]. The commonly used regimens as initial therapy in patients not eligible for transplantation are a combination of melphalan, prednisone with either of the new agents (thalidomide/ lenalidomide/ bortezomib). When novel drugs are not available, melphalan and prednisone (MP) are still used as standard therapy in elderly patients [10].
Patients who receive thalidomide have a high risk of peripheral neuropathy and thromboembolism. Hematologic toxicity (neutropenia and thrombocytopenia) is more frequently associated with lenalidomide than thalidomide [11]. Studies in Western countries comparing thalidomide-dexamethasone (thal/dex) and lenalidomide-dexamethasone (len/dex) regimes have shown that lenalidomide is more beneficial than thalidomide in newly diagnosed multiple myeloma patients [11].
There are few studies which have compared thalidomide- dexamethasone (thal/dex) and lenalidomide-dexamethasone (len/dex) in the treatment of multiple myeloma in Indian scenario. The commonly used induction regimes for multiple myeloma in our hospital are thalidomide plus dexamethasone (thal/dex) and lenalidomide plus dexamethasone (len/dex). This study was undertaken to compare the efficacy and safety observed with thalidomide-dexamethasone versus lenalidomide-dexamethasone in newly diagnosed patients of multiple myeloma in a tertiary care hospital ( Kasturba Medical College & Hospital, Manipal) in India.
To determine and compare the efficacy of thalidomide-dexamethasone and lenalidomide-dexamethasone in the treatment of newly diagnosed cases of multiple myeloma. Adverse events observed during treatment with thalidomide-dexamethasone or lenalidomide-dexamethasone in multiple myeloma
Materials and Methods
The study was carried out after obtaining approval from Institutional Ethics Committee. It was an observational study.The case record files of patients with diagnosis of multiple myeloma and met the inclusion criteria from the January 2006 to July 2011 were studied.
Selection of Subjects
Inclusion criteria
Newly diagnosed cases of multiple myeloma of either sex who received either thalidomide-dexamethasone or lenalidomide-dexamethasone treatment. Patients who received local palliative radiation for multiple myeloma prior to treatment (thal/dex or len/dex).
Exclusion criteria
Patients who received other combination of chemotherapy. Patients who received bone marrow transplant.
The diagnosis of multiple myeloma was established based on a combination of clinical, radiographic and histopathological evidence. Thirty six patients met the inclusion/exclusion criteria and data obtained was used for the analysis. Following information was collected from the patient’s file-
Patient demographics, Medical history and Investigations outcome of various treatment regimens with regard to improvement in patient’s symptoms, Hb levels, immunoglobulin levels during follow up visit. Adverse effects associated with drug treatment were also recorded.
The follow-up data was collected one week after completion of 4 cycles of chemotherapy with either thalidomide-dexamethasone or lenalidomide-dexamethasone.The data thus obtained was analyzed on the basis of patients who achieved complete, partial or minimal response in terms of serum immunoglobulin level. The efficacy of pharmacotherapy was recorded as per patient’s response of relief or no relief of symptoms, change in Hb and serum immunoglobulin levels from baseline values at diagnosis. Toxicity profile was assessed by recording the adverse effects associated with thal/dex and len/dex treatment.
Statistical Analysis
Statistical analysis was primarily descriptive. Comparisons between categorical variables were done using chi-square test, with the level of significance set at p < 0.05. Mean change in Hb levels, total leucocyte count, platelet count and mean change in serum immunoglobulin levels after treatment was analyzed by using ANCOVA, with the level of significance at p < 0.05.
Results
The case records of 36 patients diagnosed with multiple myeloma who were treated with thalidomide/dexamethasone or lenalidomide/dexamethasone regimen were studied.
The chemotherapy dosage schedules was as follows:
Thalidomide-dexamethasone regimen (4 cycles):
Tablet Thalidomide 200mg oral daily
Injection Dexamethasone 40mg is administered intravenously on Day 1-4, Day 9-12, Day 17-20 of 1st & 3rd cycle and Day 1-4 of 2nd and 4th cycle.
Lenalidomide-dexamethasone regimen (4 cycles)
Tablet Lenalidomide 25 mg oral Day 1 – 21 of each cycle.
Injection Dexamethasone 40mg is administered intravenously on Day 1, Day 8, Day 15 and Day 21of each cycle.
Treatment distribution
Out of 36 patients with multiple myeloma in this study, 17 (47.2 %) patients were treated with thalidomide-dexamethasone and 19 (52.8 %) were treated with lenalidomide-dexamethasone.
Patient demographics
The mean age of males was 60.5±11.42 y and that of female patients was 58.7±10.87 y at the time of diagnosis of multiple myeloma in the study. The mean age of the patients was 58.7±12.47 y and 60.7±9.89 y in thal/dex and len/dex regime treated cases respectively. Out of 36 patients, 21(58.3%) were males and 15 (41.7%) were females. The male to female ratio was 1.4:1. Out of 17 patients on thal/dex, 12 (70.6%) were male and 5 (29.4%) were female. Out of 19 patients on len/dex, 9 (47.4%) were male and 10 (52.6%) were female.
Clinical features of patients with various treatment groups
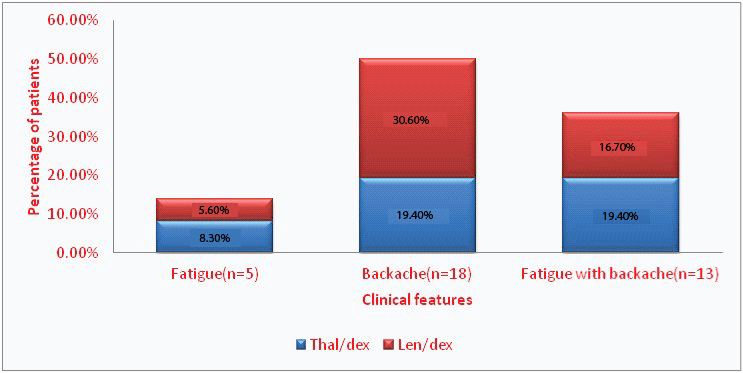
The common clinical features at time of diagnosis in patients with multiple myeloma who were in the study were backache in 18 (50%), fatigue with backache in 13 (36.1%) and fatigue in 5 (13.9%). The clinical features of patients in various treatment groups are shown in [Table/Fig-1].
Investigations
Radiological findings were present in 29 (80.6%) patients in the study. Out of 17 patients who received thal/dex, 16 (94.1%) patients and 13 (68.4%) patients out of 19 patients who received len/dex, had radiological findings suggestive of multiple myeloma.
Out of 36 patients in the study, 28 (77.8%) patients showed presence of M band on protein electrophoresis. Twelve (70.6%) out of 17 patients who received thal-dex and 16 (84.2%) out of 19 patients who received len-dex showed presence of M band on serum protein electrophoresis.
Hematology parameters at diagnosis (before treatment) [
Table/Fig-2]
Hematology parameters at diagnosis and after treatment
| Hematology | Thal/dex At Diagnosis | Thal/dex Adjusted* means ± SEM (After treatment) | Len/dex (At Diagnosis) | Len/dex Adjusted* means ± SEM (After Treatment) | p-value | 95%CI |
|---|
| Hemoglobin levels (g/dl) | 9.26± 2.8 | 10.9± 0.329 | 11.36± 2.0 | 10.9± 0.310 | 0.997 | -0.958,0.961 |
| Total leukocyte count (cells per cu.mm) | 6576.5 ± 2319.1 | 6227.1± 544.2 | 7294.7 ± 2074.1 | 5891.5± 514.4 | 0.659 | -1198.5,1869.5 |
| Platelet count (per cu.mm) | 198705.6 ± 126856.6 | 222031.4± 19473.9 | 199526.3 ± 44845 | 191766.7± 18420.5 | 0.267 | -24272.2,84801.7 |
*Adjusted for baseline
The mean hemoglobin value of patients in the study at diagnosis was 10.4 ±2.6 g/dL.The minimum and maximum hemoglobin level was 5.6g/dL and 15.9g/dL respectively. Anaemia which developed during or following treatment was observed in 29 (80.6%) patients. The hematology parameters are presented in the [Table/Fig-2].
Using ANCOVA, it was observed that there was no significant difference between thalidomide-dexamethasone and lenalidomide/dexamethasone groups after receiving treatment with respect to hemoglobin, total leucocyte count and platelet count.
Albumin globulin levels at diagnosis
| Minimum(g/dL) | Maximum(g/dL) | Mean ± SD(g/dL) |
|---|
| Albumin | 2.4 | 4.3 | 3.3±0.5 |
| Globulin | 2.7 | 10 | 5.8 ± 1.7 |
At diagnosis, the mean total protein level at diagnosis was 9.1±1.5 g/dL with minimum level being 6.10g/dL and maximum level being 12.40 g/dL. The serum globulin levels were elevated compared to serum albumin levels and also indicated a reversal in serum albumin and globulin levels which is observed in multiple myeloma.
Out of 36 patients, 21 (58.3%) patients had received palliative radiotherapy prior to start of treatment. Eight (47.1%) patients later received thalidomide-dexamethasone and 13 (68.4%) patients later received lenalidomide-dexamethasone treatment regimen.
Symptom relief during follow up visit (after 4 cycles of chemotherapy)
All the patients in the study experienced symptomatic relief after treatment as per clinician’s opinion.
Serum immunoglobulin levels before and after treatment [
Table/Fig-4,
5]
Serum Immunoglobulin levels before and after treatment
| Mean immunoglobulin levels ± SD (mg/dl) At diagnosis | Adjusted means ± SEM (mg/dl) After treatment | p-value |
|---|
| Thal/dex | Len/dex | Thal/dex | Len/dex | 0.391 |
| 4999.1±2926.6 | 3736.4±1586.1 | 1864.7±163.6 | 1665.4±134.4 |
Response among treatment groups in terms of serum immunoglobulin levels
| Treatment | Minimal response | Partial response |
|---|
| Thalidomide-dexamethasone | 5(29.4%) | 12(70.6%) |
| Lenalidomide-dexamethasone | 7(36.8%) | 12(63.2%) |
| Total | 12(33.3%) | 24(66.7%) |
The mean immunoglobulin levels at diagnosis in patients who later received thal/dex was 4999.1 ± 2926.6 mg/dL and 3736.4 ±1586.1mg/dL in patients who later received len/dex. The mean immunoglobulin levels after adjusting the values to baseline was 1864.7 ± 163.6 mg/dL and 1665.4 ±134.4 mg/dL after treatment with thal/dex and len/dex respectively.
Using ANCOVA, it was observed that there was no significant difference between therapy with thal-dex and len-dex in decreasing serum immunoglobulin after adjusting the serum immunoglobulin values to baseline.
A 50% reduction in serum immunoglobulin values from baseline after receiving treatment was considered as partial response and < 25% reduction in serum immunoglobulin values from baseline after receiving treatment was considered as minimal response. Twenty four (66.7%) patients out of 36 patients attained partial response and 12 (33.3%) patients attained minimal response in the study. Twelve patients each had partial response in thal-dex and len-dex treated group [Table/Fig-5].
Bone marrow findings after treatment
Myeloma in remission related to bone marrow was observed in 2(5.5%) patients who received treatment – one each in thal-dex and len-dex treated group. Remission was not achieved in 19 patients of which 9 (52.9%) and 10 (52.6 %) patients had received thal-dex and len-dex respectively. Remission in bone marrow was defined as absence of myeloma cells or less than 5% plasma cells on a bone marrow biopsy. Bone marrow reports after treatment were not available for 15 (41.7%) patients.
Adverse events
The adverse events which occurred during the study period have been summarised in the [Table/Fig-6]. There was no significant difference in the adverse events observed between patients who received thal-dex or len-dex.
Adverse effects related to the therapy
| Adverse effects | Thal/dex (n=17) | Len/dex (n=19) | Chi. square , p-value |
|---|
| Nausea/vomiting | 1 (5.9%) | 2(10.5%) | χ2 =0.253,p=0.615 |
| Fever | 2(11.8 %) | 0 (0%) | χ2 =2.367,p=0.124 |
| Anemia | 9(52.9%) | 6(31.6%) | χ2 =1.685,p=0.194 |
| Leucopenia | 1(5.9%) | 0(0%) | χ2 =1.150,p=0.284 |
| Thrombocytopenia | 1(5.9%) | 1(5.3%) | χ2 =0.007,p=0.935 |
| Peripheral neuropathy | 4(23.5%) | 1(5.3%) | χ2 =2.503,p=0.114 |
| Venous thrombosis | 3(17.6%) | 0(0%) | χ2 =3.658,p=0.056 |
| Constipation | 4(23.5%) | 2(10.5%) | χ2 =1.092,p=0.296 |
| Diabetes mellitus after therapy | 3(17.6%) | 1(5.3%) | χ2 =1.393,p=0.238 |
Discussion
In newly diagnosed multiple myeloma patients, randomized studies have shown that the thal/dex regimen is better than high-dose dexamethasone alone [12,13]. A prospective randomized study confirmed the efficacy of the thal/dex regimen in comparison with the standard VAD regimen [11]. Lenalidomide, an analog of thalidomide, is highly active with different toxicity profile and potentially safer than the parent drug. Trials conducted with lenalidomide plus dexamethasone in newly diagnosed multiple myeloma patients showed improved activity more than historic controls with lower toxicity in a phase 2 clinical trial [14,15].
This study examined the efficacy and safety profile of two commonly prescribed treatment regimes i.e. thalidomide/ dexamethasone and lenalidomide / dexamethasone in patients diagnosed with multiple myeloma who attended a tertiary care hospital. Of the 36 patients in this study, 17 patients had received thal-dex and 19 patients had received len-dex.
As per literature available, the mean age of affected individuals is 62 y in men and 61 y for women [16–18]. We had similar findings in our study. Male preponderance has been observed among multiple myeloma patients [16–18]. The male to female ratio in our study was 1.4:1. Backache due to pathological vertebral fractures, fatigue with backache, fatigue, presence of radiological findings like bone erosions and presence of M band at diagnosis of multiple myeloma were noted in this study. At the time of diagnosis, majority of the patients were anaemic. These results are in concordance with a study conducted by Kyle et al., at Mayo Clinic, Minnesota in which newly diagnosed multiple myeloma patients were observed to determine clinical and laboratory features [16,17].
A study conducted by Gay F et al., in 411 newly diagnosed multiple myeloma patients in Mayo Clinic showed that higher proportion of patients achieved partial response with len/dex compared to thal/dex regimen [11]. Partial response was observed in majority of patients in our study. No significant difference was observed in terms of partial response related to serum immunoglobulin levels or bone marrow remission between the two treatment groups in this study. There was no significant difference between treatment arms with respect to hematology parameters after receiving treatment in our study. In our study, there was no significant difference between thal/dex and len/dex regimens in terms of efficacy.
In the study conducted by Gay F et al., more adverse events were observed with patients receiving thal/dex as compared to len/dex treatment. Incidence of thromboembolic events and peripheral neuropathy was observed more in thal/dex group. Len/dex treatment was associated with neutropenia. Lenalidomide was well-tolerated and more effective than thal/dex in the study conducted by Gay et al. Though the results show that thal/dex treatment is associated with more adverse events as compared to len/dex regimen, there was no significant difference among the treatment groups in causing any specific adverse event. There was no significant difference between thal/dex and len/dex regimens in terms of adverse events in our study. There are some limitations of the study. The sample size was small. Moreover, patients were evaluated only once - a week after completion of four cycles of chemotherapy. Long term follow up data was not collected.
Conclusion
There was no significant difference between thal/dex and len/dex treatment groups with respect to efficacy and safety in our study. Studies with larger sample size and a longer follow up to compare efficacy and safety of thal/dex and len/dex in treatment of multiple myeloma are required to be carried out to provide significant results. There has been a major shift in the treatment of multiple myeloma in the last decade. Better understanding of the underlying process of malignant transformation and tumour propagation ultimately should enable the development of more efficient therapy and better outcome of patients.