Even after practicing endodontic protocol for centuries, cent percent success seems like a mirage, a 14-18% of failure has been observed [1]. There is an increasing demand to preserve teeth, and a growing interest in conventional retreatment. This procedure requires the removal of the existing obturation, further instrumentation, disinfection and re-obturation [2]. Removing the maximum amount of obturating material from inadequately prepared and/or obturated root canal system appears to be essential in order to uncover remaining necrotic tissue or bacteria that may be responsible for the persistent disease and enable thorough chemomechanical re-instrumentation and disinfection of the root canal system [1,3,4].
One of the various techniques for facilitating removal of old endodontic obturation, is chemical dissolution of gutta percha using gutta percha solvents. Chloroform, eucalyptol, orange oil, halothane are some of the examples of gutta percha solvents. There is not much literature available for evaluating the role of solvents in cleanliness of dentinal tubules after retreatment using SEM. Horvarth et al., concluded that solvents led to more gutta percha and sealer on root canal walls and inside dentinal tubules [2]. It was proposed that further studies should evaluate the effect of ultrasonic irrigation on the cleanliness of dentinal tubules during endodontic retreatment. Till now, the effect of ultrasonic irrigation alone and in combination with chloroform has not been assessed for evaluating cleanliness of dentinal tubules using scanning electron microscope (SEM). Hence, an in vitro study was framed to evaluate the dentinal tubules visually by SEM if root filling material remained in dentinal tubules after gutta percha removal was done with and without using chloroform and ultrasonic irrigation.
Materials and Methods
Specimen preparation- Freshly extracted 45 human mandibular premolars for periodontal and orthodontic reasons were used in the study. The study was designed and executed in the Department of Conservative Dentistry and Endodontics at Institute of Dental Sciences, Bareilly in 2012, after obtaining clearance from the institutional ethical committee. Conventional radiographs were taken at two different angulations for evaluation and to exclude teeth with caries, fractures, calcification and multiple canals. Teeth which were caries free, having a single canal and a straight root were included in the study. Specimen length was measured using a vernier caliper. The selected teeth were at least 20 mm long. Teeth with patent canals and canal curvature angles varying between 0–10° as given by Schneider [5] were selected, after radiographic evaluation.
All teeth were stored in 10% ethyl alcohol solution. Access cavity preparations were done and the incisal edges were adjusted, so that the final working length of each tooth was 19.5 mm. The working length was confirmed by radiographs. Radiographs were taken to confirm that the distance of file from the apical foramen remained between 0.5-1mm in all the specimens.
Canal preparation- All the roots were instrumented using K- type file (Dentsply, Maillefer, Ballaigues, Switzerland). The apical enlargement was done up to size 40 using K file at the working length by using the files in sequence according to increasing order of their tip diameter size (size 15-40 K file). Frequent recapitulation was done by using number 15 K file. A step back technique was followed for cleaning and shaping of the canal. K files of sizes 45,50,55 were used in progressing order at file lengths 1 mm short of the preceeding file. (i.e. at 18.5mm,17.5mm and 16.5mm for K file 45,50 and 55 respectively). K file size 40 was used for recapitulation to prevent iatrogenic ledge formation. Using side vented needles (canal clean), 3% sodium hypochlorite was delivered in the root canals each time before using instrument of larger diameter. Finally, the root canals were rinsed for 1 minute using EDTA, followed by 3% NaOCl (10 ml) for final rinse. A 28-gauge side vented irrigation needle, inserted 1–2 mm short of the working length was used for irrigation. All root canals were dried with paper points.
Obturation- All samples were randomly divided into three groups. Group 1: (Control Group, n = 5) The roots remained unobturated and it served as a baseline parameter for comparison. The root canal of each tooth in experimental Groups 2 and 3 were obturated using lateral compaction. The roots were radiographed in buccolingual and mesio-distal directions in order to confirm the adequacy of the obturation. The access cavities were filled temporarily by Cavit (3M ESPE). All teeth were stored in a humid or for two weeks in 100% humidity to allow complete setting of the sealer.
Retreatment technique- In Groups 2 and 3, from the coronal 5 mm of the root canal of each specimen the obturating material was removed using Gates Glidden drills of sizes 2, 3 and 4 in sequential increasing order of their size. In the middle and apical part of the canal, Hedstrom files sizes 15-40 (Dentsply, Maillefer, Ballaigues, Switzerland) were used in order to remove gutta-percha and sealer from the canal. In Group 2, chloroform (Rankem, Ranbaxy), a gutta percha solvent was used along with H files to ease the removal of gutta percha. In Group 3 also gutta-percha removal was done by using H files without using chloroform.
In Group-2 chloroform was deposited for 15 sec into the reservoir created by Gates Glidden drill. The gutta-percha was removed with Hedstrom files sizes 40–15 (in descending order) to the working length using a push and pull action. Once the working length had been reached with a size 15 file, sizes 20, 25, 30, 35, 40 were instrumented to the working length. When no gutta-percha could be seen on the flutes of the file, radiographic confirmation was done and the gutta-percha removal was ceased. After gutta-percha removal, specimens from both the groups were divided in two sub groups. In subgroup I (n=10), the canals were irrigated with 3% NaOCl (10ml) for one minute using side vented needles 1-2mm short of working length. In subgroup II (n=10), canals were subjected to passive ultra sonic irrigation by ultrasonic file along with 3% sodium hypochlorite for one minute. Finally, all canals were dried with paper points (Dentsply, Maillefer, Ballaigues, Switzerland).
Evaluation-The teeth were grooved with a diamond saw and split longitudinally using chisel and mallet. For the SEM analysis, the specimens were dehydrated with ascending concentrations of ethyl alcohol (30-100%) and then sputtered with gold. The root halves were examined using a SEM at 10–15 kV and at a standard magnification of 2000 X. Each root half was evaluated by an observer who was blinded to which technique was used for the removal of the gutta-percha. Evaluation was done for three different locations i.e. coronal, middle and apical third. For statistical analysis, the total number of dentinal tubules and the number of dentinal tubules either completely or partially occluded with obturating material were recorded.
Statistical Analysis
A one-way ANOVA test was performed to calculate the mean value and standard error in each group for each third of canal for occluded tubules over total tubules. Normality of error terms can be assumed. In the analysis, all observations were included distinguishing between the coronal, middle and apical third. The group effect was calculated and the p-values for the pair wise comparisons were adjusted using Tukey’s test. Significance was established at 1% (p< 0.01).
Results
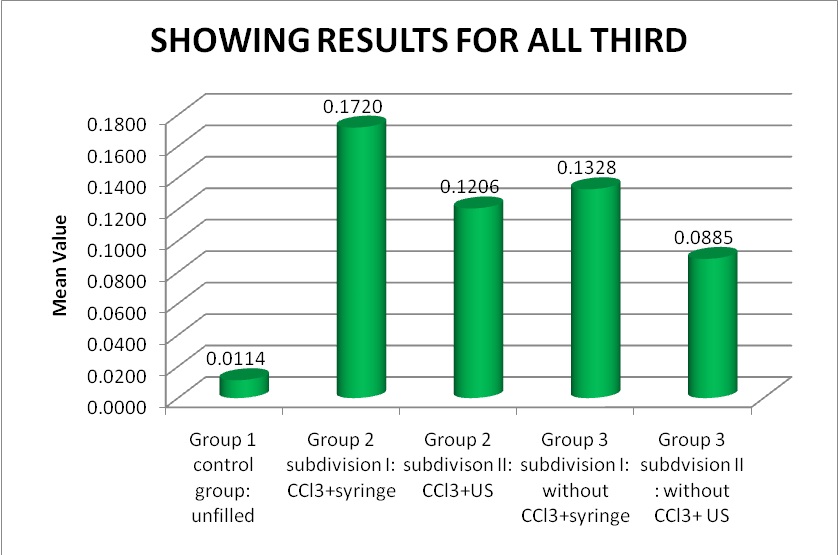
After combining the mean values of occluded tubules/total number of dentinal tubules of all thirds of the canal, the cleanest dentinal tubules was found in following order: Group 1> Group 3 subdivision II> Group 2 subdivision II> Group 3 subdivision I> Group 2 subdivsion I [Table/Fig-1,2]. [Table/Fig-3a,b,c,d,e] shows representative SEM images from all groups.
Scanning Electron Microscope (SEM) analysis of specimen
| For coronal third |
|---|
| Group | n | RANGE | MEAN ± SD | SE |
|---|
| Group 1 control group: unfilled | 5 | 0.0091-0.0167 | 0.012199 ± 0.00299 | 0.0013385 |
| Group 2 subdivision I: CCl3+syringe | 10 | 0.1476-0.1980 | 0.174593 ± 0.01630 | 0.0051557 |
| Group 2 subdivison II: CCl3+US | 10 | 0.0889-0.1238 | 0.113396 ± 0.01110 | 0.0035108 |
| Group 3 subdivision I: without CCl3+syringe | 10 | 0.1220-0.1324 | 0.126650 ± 0.00357 | 0.0011313 |
| Group 3 subdvision II : without CCl3+ US | 10 | 0.0837-0.1136 | 0.091089 ± 0.00911 | 0.0028812 |
| For middle third | | | | |
| Group 1 control group: unfilled | 5 | 0.0000-0.0145 | 0.006879 ± 0.00654 | 0.0029282 |
| Group 2 subdivision I: CCl3+syringe | 10 | 0.1524-0.1850 | 0.172511 ± 0.01049 | 0.0033196 |
| Group 2 subdivison II: CCl3+US | 10 | 0.1095-0.1378 | 0.118208 ± 0.00791 | 0.0025044 |
| Group 3 subdivision I: without CCl3+syringe | 10 | 0.1280-0.1422 | 0.137183 ± 0.00501 | 0.0015856 |
| Group 3 subdvision II : without CCl3+ US | 10 | 0.0837-0.0977 | 0.090822 ± 0.00446 | 0.0014114 |
| For apical third | | | | |
| Group 1 control group: unfilled | 5 | 0.0098-0.0222 | 0.015011 ± 0.00506 | 0.0022647 |
| Group 2 subdivision I: CCl3+syringe | 10 | 0.1535-0.1832 | 0.168829 ± 0.01053 | 0.0033304 |
| Group 2 subdivison II: CCl3+US | 10 | 0.1116-0.1698 | 0.130161 ± 0.01553 | 0.0049129 |
| Group 3 subdivision I: without CCl3+syringe | 10 | 0.0952-0.1476 | 0.134551 ± 0.01499 | 0.0047431 |
| Group 3 subdvision II : without CCl3+ US | 10 | 0.0744-0.0896 | 0.083622 ± 0.05821 | 0.0018409 |
| For all third | | | | |
| Group 1 control group: unfilled | 15 | 0.0063-0.0149 | 0.011363 ± 0.00389 | 0.0017422 |
| Group 2 subdivision I: CCl3+syringe | 30 | 0.1512-0.1812 | 0.171987 ± 0.00942 | 0.0029801 |
| Group 2 subdivison II: CCl3+US | 30 | 0.1135-0.1314 | 0.120588 ± 0.00580 | 0.0018347 |
| Group 3 subdivision I: without CCl3+syringe | 30 | 0.1181-0.1386 | 0.132795 ± 0.00589 | 0.0018648 |
| Group 3 subdvision II : without CCl3+ US | 30 | 0.0849-0.0955 | 0.088511 ± 0.00364 | 0.0011519 |
Estimated mean, standard deviation (SD), standard error (SE), of the ratio evaluated in SEM (number of occluded dentinal tubules/total dentinal tubules) analysis and number of evaluated images (N) CCl3=chloroform US=ultrasonic
Depicting ratio of occluded/total number of dentinal tubules of all specimens
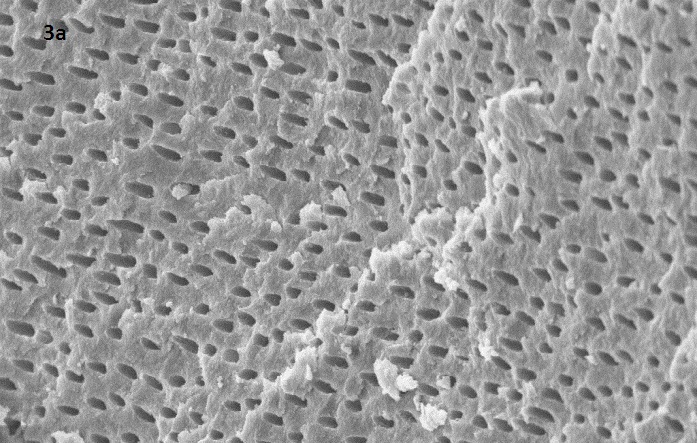
SEM image of Group 1(control group)
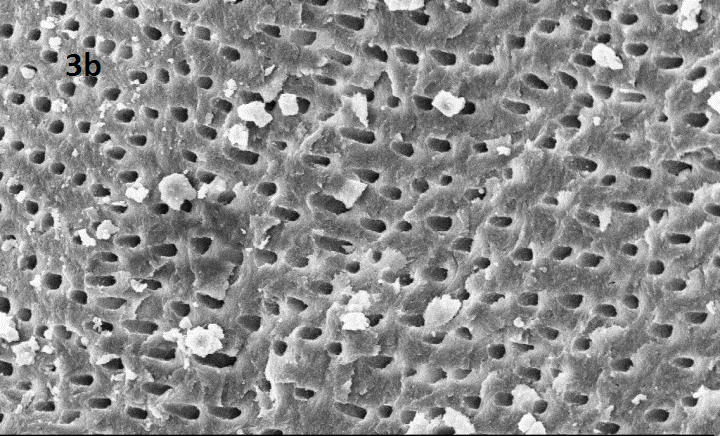
SEM image of group 2 subdiv I: CCl3 + syringe
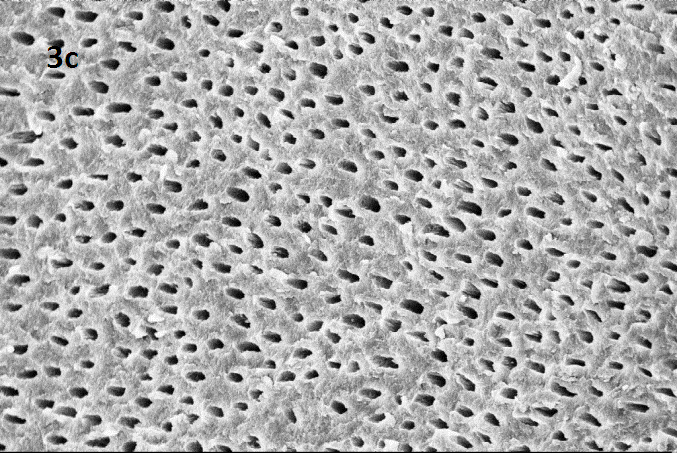
SEM image of Group 2 subdiv II: CCl3 + US
SEM image of group 3 subdiv I: without CCl3 + syringe
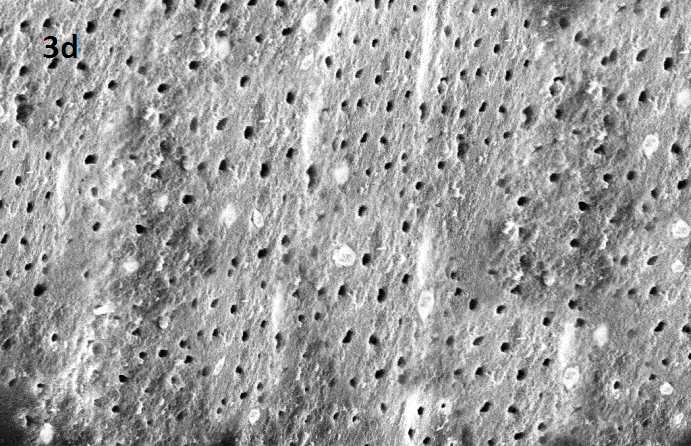
SEM image of Group 3 subdiv II: without CCl3 + US
Representative SEM images at magnification 2.00 KX for each group (1: control group, 2 subdiv I: CCl3 + syringe , 2 subdivII :CCl3 + US ,3 sub divI :without CCl3 + Syringe, 3 subdiv II : without CCl3 + US)

Discussion
The most important factor associated with endodontic failure is the persistence of microbial infection in the root canal system and/or the periradicular area. Bacteria located in areas such as isthmuses, ramifications, delta’s, irregularities and dentinal tubules may be seldom not affected by endodontic disinfection procedures [6]. In such anatomical regions, bacteria entombed by the root filling usually die or are prevented from gaining access to the periradicular tissues. If the root canal filling fails to provide a complete seal, seepage of tissue fluids can provide substrate for bacterial growth. In such cases the endodontic treatment often fails and requires retreatment. To prevent failure, it is necessary that disinfectant and delivery system is chosen wisely to ensure its availability and wettability in the uninstrumented areas. To improve the wettability of irrigant, various agitation techniques have been developed, like manual brushes, rotary brushes, ultrasonic and sonic devices and pressure alternating devices [7]. However, using hand held syringe needle (both open ended and side vented) irrigation may show more cases of unsuccessful root canal treatment due to weak mechanical flushing of debris [4].
According to a survey by Ravanshad S. despite of introduction of various newer techniques the most commonly used protocol by majority of dentists in endodontic therapy is use of hand files for instrumentation, use of sodium hypochlorite as chief irrigant, use of syringe for delivery of irrigant and use of cold lateral condensation technique for obturation [8]. Dunter reported that passive ultrasonic irrigation is used as second most commonly used irrigation system after syringe irrigation in USA as per a survey done in the year 2011 [9]. Due to the above reasons, the study incorporated cold lateral condensation as an obturation technique and compared passive ultrasonic irrigation against syringe irrigation for their efficacy in retreatment.
Previous data suggests that further studies should be conducted to evaluate the effect of ultrasonic irrigation on the cleanliness of dentinal tubules during endodontic retreatment [2]. Therefore, prior to this study, another study was planned which compared ultrasonic irrigation and syringe irrigation for the area covered by root filling material in the apical third of canal wall after retreatment. The evaluation was done pictographically for the apical third of canal wall and result indicated significantly cleaner canal when ultrasonic irrigation was used [10]. Since pictographic evaluation was limited to evaluating clean canal surface area, further research was needed for deeper insight on cleanliness of number of dentinal tubules. Hence, this study used SEM for evaluating the influence of passive ultrasonic irrigation in cleanliness of dentinal tubules for all thirds of canal wall. This study also studied influence of chloroform, a gutta-percha solvent on cleanliness of dentinal tubules as chloroform plays an integral part in retreatment cases.
Cameron postulated that there is a synergistic effect between sodium hypochlorite (NaOCl) and US (ultrasonic irrigation) [1] and it being the most commonly used irrigant, it was used in the present study. Narrow canals may compromise the effectiveness of ultrasonic irrigation because when sonic or ultrasonic files are used in small, curved canals, they may bind, thus restricting their vibratory motion and cleaning efficacy [11,12]. Therefore during the specimen preparation, the specimen were adequately prepared to an apical size 40 K file and root canal were flared using K files from sizes 45-55 using step back technique. This allowed needle/cannula used for delivery or irrigant to remain loose inside the canal during irrigation and also allows the irrigant to reflux and facilitate more debris to be displaced coronally, while avoiding the inadvertent expression of the irrigant into periapical tissues [6]. Previous studies compared H files against various rotary files systems for removal of root canal filling material and it was found that no single system lead to significantly cleaner canal, H files were considered to be chosen for this study to minimize the chances of instrument breakage as seen with rotary instruments during gutta percha retrieval [13,14].
Chloroform was used as gutta percha solvent as it is the most popular solvent because of its organic nature which solubilizes gutta-percha more rapidly than eucalyptol oil [15]. It is less expensive, is easy to obtain, and has a more pleasant odour [16]. Amount of chloroform normally used in endodontics is insignificant and poses no health hazard [17].
The concept of using ultrasonics in endodontics was first introduced by Richman [18]. The term endosonics was coined by Martin and Cunningham and was defined as the ultrasonic and synergistic system of root canal instrumentation and disinfection [19,20]. It has been demonstrated that an irrigant in conjunction with ultrasonic vibration, which generates a continuous movement of the irrigant, is directly associated with the effectiveness of the cleaning of the root canal space [21,22]. Acoustic streaming, as described by Ahmad et al., has been shown to produce sufficient shear forces to dislodge debris in instrumented canals [23]. The flushing action of irrigants may be enhanced by using US. This seems to improve the efficacy of irrigation solutions in removing organic and inorganic debris from root canal walls [24]. A possible explanation for the improved action is that a much higher velocity and volume of irrigant flow is created in the canal during ultrasonic irrigation. US create both cavitation and acoustic streaming [25]. Acoustic streaming is the rapid movement of fluid in a circular or vortex-like motion around a vibrating file. Cavitation in the fluid mechanical context can be described as the impulsive formation of cavities in a liquid through tensile forces induced by high-speed flows or flow gradients. These bubbles expand and then rapidly collapse producing a focus of energy [26].
The above properties of ultrasonic irrigation might be the reason of cleaner dentinal tubules in the specimen which were subjected to ultrasonic irrigation as compared to those subjected to syringe irrigation. A recent study which evaluated efficacy of xylene and passive ultrasonic irrigation on remaining root filling material during retreatment also established enhanced removal of filling materials when ultrasonic irrigation was used [27].
In the present study, the roots were split and evaluated under scanning electron microscope (SEM) under a constant magnification of 2000 X. The number of filled dentinal tubules was evaluated for the coronal, middle and apical third of each root half. The calculations were tabulated and mean values were compared using one-way ANOVA test. Control group in which canals remained unfilled served as baseline parameter for comparison.
It was deduced that group in which chloroform was not used lead to cleaner dentinal tubules irrespective of irrigation type, meaning thereby subdivision II>I in both Group 3 (.08 Vs .13) and Group 2 (.13 Vs .16). It seems that more remnants were found in irregularities of the rootcanal wall and in dentinal tubules with increasing dissolution of the root filling material. This might be explained by the fact that softened root obturating material may easily be compacted into these irregularities and into dentinal tubules from where they can no longer be removed. However use of ultrasonic irrigation lead to significantly cleaner dentinal tubules than syringe irrigation in both the groups. Group 2 (.13 Vs .16) and Group 3 (.08 Vs .13) i.e. sub division II>I. This can be attributed to its property of acoustic streaming and cavitation. Also on comparing cleanliness in ultrasonic group in all the thirds the apical third showed cleanest tubules based on the fact that acoustic micro streaming depends inversely on the surface area of the file touching the root canal wall. Ultrasonic irrigation showed better results than syringe Group 2 and 3 (sub division II>I) due to weak mechanical flushing action of syringe and inability to reach up to working length in the canal [4].
Limitations of syringe irrigation can be attributed to weak mechanical flushing, inaccessibility in irregularities of root canal walls, inability of irrigant delivery beyond 1mm from tip of needle. In contrast to syringe irrigation, phenomenon of acoustic streaming and cavitation is seen in passive ultrasonic irrigation [6]. It could be inferred from the results that ultrasonic irrigation if followed by removal of filling material leads to significantly cleaner canals and use of chloroform leads to compaction of softened gutta percha into dentinal tubules therefore to achieve clean dentinal tubules chloroform should not be used in removal of gutta percha. Hence in retreatment cases use of ultrasonic irrigation is advantageous and chloroform should be best avoided in removal of gutta percha.
Conclusion
Under the limitations of this study it could be concluded that both ultrasonic and syringe irrigation showed cleaner canals when chloroform was not used. Hence, chloroform should be utilized only when mechanical methods fail to achieve retrieval of gutta percha in retreatment cases. Irrigation when done with ultrasonics leads to cleaner tubules than syringe irrigation. Hence, mechanical methods of retrieval in conjunction with use of passive ultrasonic irrigation should be a part of retreatment protocol.
Estimated mean, standard deviation (SD), standard error (SE), of the ratio evaluated in SEM (number of occluded dentinal tubules/total dentinal tubules) analysis and number of evaluated images (N) CCl3=chloroform US=ultrasonic