Background: Hypercholesterolemia is a major risk factor for cardiovascular disease. Not all patients respond well to traditional cholesterol lowering medications. Probiotics have been evaluated for their cholesterol-lowering effects in humans with variable results. This study was performed to evaluate the efficacy of two probiotics in lowering the serum cholesterol of hypercholesterolemic patients.
Materials and Methods: A randomized double-blind controlled trial was conducted comparing placebo to Lactobacillus acidophilus plus Bifidobacterium bifidum in patients diagnosed with hypercholesterolemia. Placebo or probiotic capsules were taken three times daily for six weeks. Pre- and post-treatment total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C) and triglyceride (TG) levels and demographic parameters of the two groups were compared. From a total of 70 participants, 64 completed the assigned treatment (31 in probiotics group and 33 in the control group).The two treatment groups were matched for age, sex, weight, height, BMI, waist and hip circumferences, and blood pressure.
Results: Baseline evaluation revealed no difference between the probiotics group and control group levels of TC, HDL-C, LDL-C and TG. TC levels in the probiotics group decreased during treatment (237.2 vs. 212.7 mg/dL, p<0.05). TC and LDL-C levels in the control group increased significantly from their baseline levels during treatment. TC (212.7 vs 252.8 mg/dL, p<0.001), HDL-C (52.0 vs 59.1 mg/dL, p=0.04) and LDL-C (153.9 vs 182.1 mg/dL, p<0.01) levels in the probiotics group were significantly lower at the end of treatment than the corresponding levels in the control group.
Conclusion: A combination of Lactobacillus acidophilus and Bifidobacterium bifidum decreased serum total cholesterol, LDL-cholesterol and HDL-cholesterol levels in hypercholesterolemic patients over a six week period. There was no effect on serum triglyceride or fasting blood glucose levels.
Introduction
Hypercholesterolemia is a major risk factor for coronary artery disease and myocardial infarction [1,2]. Recent recommendations for lowering blood cholesterol include dietary management, behavioural modifications, exercise and medications [3,4]. These measures are not always effective in controlling blood cholesterol levels. Some measures, such as pharmacological treatment, can be associated with adverse side effects [5].
Probiotics are widely used to promote health in human subjects. The potential usefulness of probiotics for lowering cholesterol levels has been explored. Lactobacillus and bifidobacterium are the most widely used probiotics of the lactic acid-producing bacteria [6]. The proposed mechanism of action of lactobacilli and bifidobacteria for lowering cholesterol is to deconjugate bile acids and increase their rate of excretion [7]. Cholesterol is a precursor of bile acids and is metabolized to bile acid as they are excreted, resulting in a reduction of serum cholesterol. Several animal studies have demonstrated the efficacy of probiotics, in the form of fermented milk or specific strains of lactic acid bacteria, in lowering cholesterol [8-12]. Studies in humans have had conflicting results [12-20]. There is no study using the combination of Lactobacillus acidophilus and Bifidobacterium bifidum as probiotics for lowering blood cholesterol. The current study was conducted to investigate the efficacy of a two-strain combination probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) in lowering blood cholesterol in healthy adults with hypercholesterolemia.
Materials and Methods
A randomized double-blind controlled trial was conducted between January and June 2013 at the Preventive Medical Clinic uppaphol2of Srinakharinwirot University Hospital, Thailand. This study was approved by the ethics committee of the Faculty of Medicine, Srinakharinwirot University (Thai Clinical Trials Registry number TCTR20130000001). Written informed consent was obtained from patients before enrollment in the study. The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Patients diagnosed with hypercholesterolemia (defined as a total cholesterol ≥200 mg/dL) during a routine healthy check-up were eligible to participate in the study. The inclusion criteria included: 1) had a repeated total cholesterol level ≥200 mg/dL prior to allocate to the study group, and 2) declined conventional lipid lowering medical treatment. Patients who: 1) had underlying diseases such as ischemic heart disease, inherited lipid metabolic disorders, chronic gastrointestinal disease, immunodeficiency, malignancy, and mental disabilities, 2) currently used any lipid lowering drugs,and 3) pregnant or lactating women were excluded from the study. Patients were allowed to withdraw from the study at any time and the reason for withdrawal was recorded. Patients were asked to avoid taking probiotics and probiotic containing products, such as fermented dairy products, during the study. From a total of 70 patients diagnosed with hypercholesterolemia, 66 were recruited into the study and were randomized to 1:1 ratio to receive either a two-strain combination probiotics or placebo by a computerized program (Graph Pad Quick Cals, Graph Pad Software, La Jolla, CA, USA) by a statistician blinded to the study. The investigators and patients were blinded to the intervention. The code to the randomization sequence was opened only after the study was complete.Participants in both groups were instructed to maintain their normal daily activity with minimal calorie and lipid intake during the study period.
We estimated that with 25 patients per group, we would be able to detect a decrease in TC level of 14.3 mg/dL in the probiotics group versus 0.8 mg/dL in the control group, with a common standard deviation of 16.6 mg/d, [21] 80% power, and a two-tailed alpha error of 0.05. In total, we planned to enrol 33 patients per group to account for a possible 30% follow-up loss.
Intervention
After enrolment, venous blood samples obtained after a 12 h overnight fast. Baseline serum total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), triglycerides (TG) and fasting blood glucose (FBG) levels were determined. Serum TC, HDL-C, LDL-C and TG were measured using an enzymatic method. Blood glucose was measured by the glucose oxidation technique. Patients with a baseline TC less than 200 mg/dL were excluded from the study. Demographic characteristics including age, sex, weight, height, waist and hip circumference and blood pressure were recorded at the beginning of the study by a nurse. Weight was measured to the nearest 100 g using an electronic scale (Seca®, Model 767, Hamburg, Germany). Standing height was measured using a height rod (Seca®, Model 220, Hamburg, Germany). Waist circumference was measured using a non-stretch tape with the subject in the standing position, at a point midway between the lower costal margin and the top of the iliac crest. Hip circumference was measured in the standing position over the maximum circumference of the buttocks. Body mass index (BMI) was calculated as the ratio of weight in kilograms divided by height in meters squared.A BMI of 25 kg/m2 or over was considered obese [22].
Patients in the probiotics group were given a probiotic containing capsule containing Lactobacillus acidophilus (minimum of 109cfu/ capsule) and Bifidobacterium bifidum (minimum of 109cfu/ capsule) (Infloran®, Berna, Switzerland) thrice daily for six weeks. Patients in the control group took placebo capsules thrice daily for six weeks, as in the probiotics group. Patients assigned to the control group were given a placebo capsule identical in size and colour to the probiotic capsule. Two weeks of capsules were provided to the patients and patients were asked to visit the clinic every two weeks with the remaining capsules in order to measure compliance. New capsules were provided to patients at every visit.
At the end of the study (week 6), blood samples were collected and were tested for TC, HDL-C, LDL-C, TG and FBG, using the same procedure as at enrolment. Demographic data of patients were recorded using the same method as at the beginning of the study. Open ended questions were used to assess side-effects of taking capsules in both groups. The sum of capsule intake was used to assess compliance.
Outcome Measurements
The primary outcome was level of total cholesterol at the end of study, compared to that at the beginning of the study. Changes in other serum lipids and fasting blood glucose levels and demographic characteristics were assessed as secondary outcomes.
Statistical Analysis
The normality of distributions of continuous variables was assessed by Kolmogorov-Smirnov test. Normally distributed variables were presented as means and standard deviations. Non-normally distributed variables were presented as medians and interquartile ranges. The Pearson chi-square or Fisher-exact test was used, where appropriate, to compare proportions between groups. Normally distributed continuous group data were compared using the student’s t-test. Non-normally distributed group data were compared using the Mann-Whitney U-test.
Outcomes at the end of study were compared to the baseline using a paired t-test and Wilcoxon signed rank test for normally distributed and non-normally distributed data, respectively. Statistical analyses were performed using SPSS software (version 16.0, SPSS, Chicago, IL, USA). P <0.05 was considered statistically significant.
Results
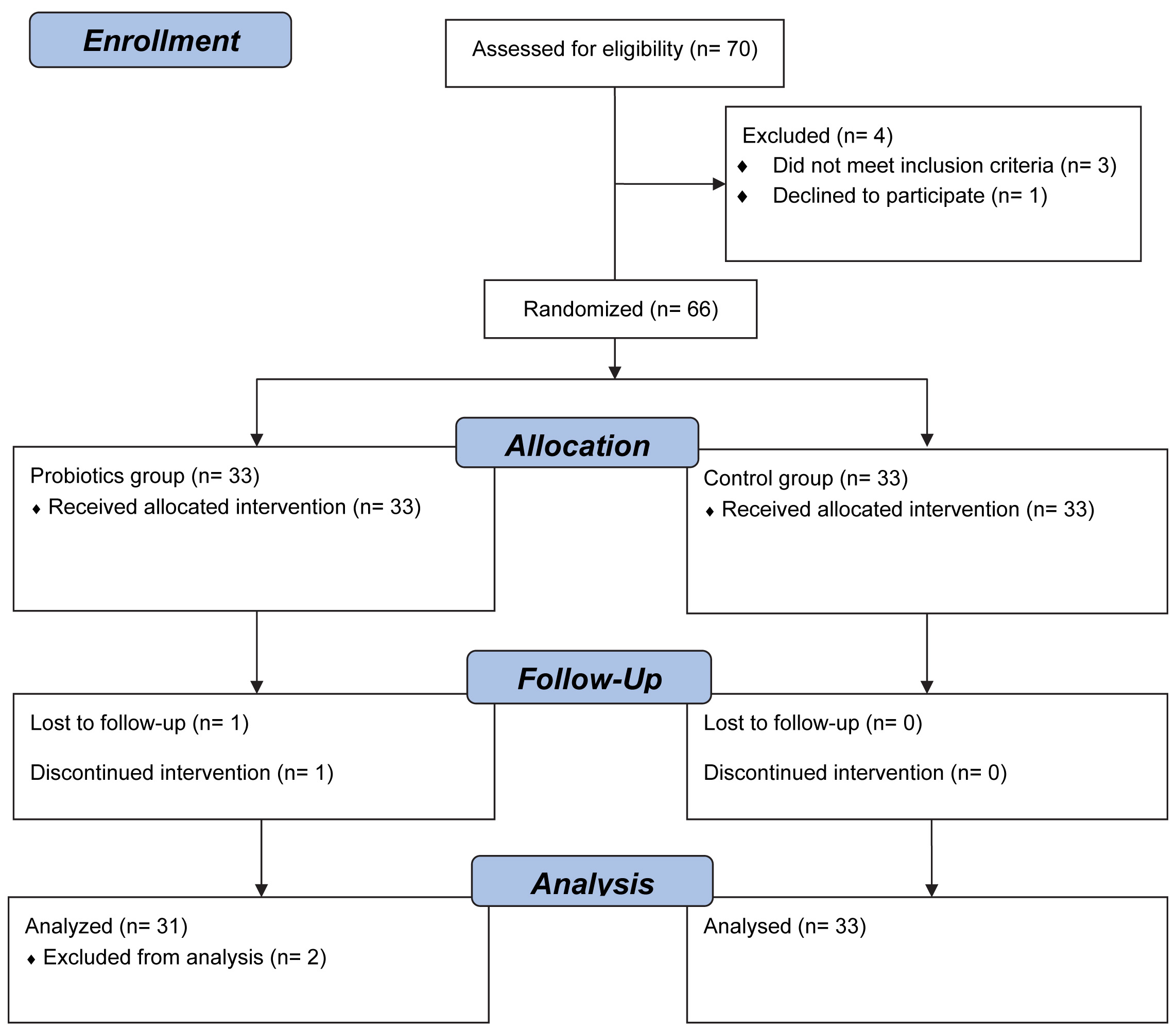
Seventy patients with hypercholesterolemia during a routine health check-up that declined standard medical treatment with lipid-lowering medications were approached to participate in this study. One subject declined to participate and another three patients were excluded from the study because a repeat blood examination before study group allocation revealed total cholesterol less than 200 mg/dL. A total of 66 patients were allocated into 2 study groups, 64 of the 66 patients completed the assigned treatment, (31 in the probiotics group and 33 in the control group [Table/Fig-1]. Two patients in the probiotics group withdrew from the study. One patient experienced dizziness after enrolment and was placed on conventional treatment with lipid-lowering medications. One patient did not return to clinic and was lost to follow up. Therefore, 64 patients were included in the final analysis.
Baseline demographic characteristics are shown in [Table/Fig-2]. Twenty-two patients (34.4%) were male. The mean age, weight and BMI of patients were 49.3±7.6 y, 67.1±9.7 kg and 26.2±4.0 kg/m2, respectively. The two treatment groups were matched for age, sex, weight, height, BMI, waist and hip circumferences, and blood pressure. The prevalence of obesity in the probiotics treatment group was comparable to that of the control group (48.4% versus 63.6%, p = 0.31). Serum lipids and FBG at the baseline and at the end of study are presented in [Table/Fig-3]. Baseline serum lipid (TC, HDL-C, LDL-C and TG) data was normally distributed and fasting blood glucose data was not normally distributed. Baseline evaluation revealed no difference in the probiotics group and control group levels of TC (237.2 vs. 231.0 mg/dL,p=0.39), HDL-C (56.5 vs 60.7 mg/dL, p=0.33), LDL-C (158.1 vs 159.4 mg/dL, p=0.87) and TG (175.9 vs 151.0 mg/dL, p=0.38). However, patients in the probiotic group had significantly higher median levels of FBG than those the in control group (100.0 vs 95.0 mg/dL, p=0.04).
Serum lipid and FBG levels of the probiotics group decreased during treatment. However, only TC was significantly lower at the end of treatment. TC and LDL-C levels of the control group increased significantly from their baseline levels during treatment. Treatment decreased TC -24.5 mg/dL (95% CI -34.7 to -14.4 mg/dL) in the probiotics group and increased TC +21.8 mg/dL (95% CI 9.4 to 32.4 mg/dL) in the control group. LDL-C in the control group increased +22.6 mg/dL (95% CI 9.4 to 35.9 mg/dL)with treatment.TC, HDL-C and LDL-C levels in the probiotics group were significantly lower at the end of treatment than The corresponding levels ofthe control group. There was no difference in the TG and FBG levels of the two treatment groups at the end of treatment. Mean differences of the TC, HDL-C and LDL-C levels of the probiotics and control groups at the end of study were -40.1 mg/dL (95% CI: -59.0 to -21.2 mg/dL, p-value <0.001), -7.1 mg/dL (95% CI: -13.9 to -0.3 mg/dL, p=0.04) and -28.2 mg/dL (95% CI: -48.9 to -7.5 mg/dL, p<0.01), respectively [Table/Fig-3].
Patient weight, BMI, and waist and hip circumference of both study groups did not change significantly over the short course of the study. In the probiotics group, systolic blood pressure decreased from 139.5±24.4 mmHg to 131.6±20.4 mmHg over the course of the study (p=0.01). Diastolic blood pressure did not change during this time interval (87.7±16.5 vs 84.0±14.9 mmHg, p=0.20). There were no significant changes in the systolic or diastolic blood pressure of the control group over the course of the study.
Compliance with capsule intake in both study groups was comparable, 94.0±9.2% in the probiotics group and 90.3±6.4% in the control group. No major adverse effects were reported except for one patient in the probiotics group who withdrew shortly after recruitment due to feelings of dizziness. Three patients (2 in the probiotics and 1 in the control group) reported loose stool and one patient in the control group reported nausea. All these events subsided uneventfully without treatment.
Discussion
This blinded randomized controlled study found that a combination Lactobacillus acidophilus plus Bifidobacterium bifidum probiotics, taken in capsule form over six weeks, had efficacy in lowering serum cholesterol in patients with hypercholesterolemia. This treatment had no efficacy in lowering serum triglyceride or fasting blood glucose levels. These findings are in agreement with the majority of previous studies evaluating the cholesterol lowering effects of probiotics.
This is the first study demonstrating the efficacy of Lactobacillus acidophilus plus Bifidobacterium bifidum in capsule form for lowering serum cholesterol in hypercholesterolemic patients. Patients in the probiotic group had a significant decrease in TC levels during treatment, while TC levels in the control group actually increased during treatment. Both animal [8-12] and human [12,14,16-20] studies have corroborated these findings, with a small number of studies showing no benefit with probiotics [13,15].These studies varied in type of probiotics administered, dosage, formulation (in foods or in capsules, single or mixed probiotics), and clinical setting (hypercholesterolemic patients or healthy individuals with normal cholesterol levels) [21]. A meta-analysis showed that probiotics decreased total cholesterol and LDL-C levels in patients with high and normal cholesterol levels, and had no significant effect on triglyceride levels [23], similar to our findings.This meta-analysis did not find any improvement in HDL-C levels associated with probiotics treatment, also similar to our findings. A decrease in HDL-C levels of -5.4 mg/dl (95% CI:-10.33 to -0.47) has been reported in probiotics treated patients [24], similar to the decrease we observed.
TC, LDL-C and HDL-C levels in the probiotics group were significantly lower than those in the control group after 6 weeks of treatment. LDL-C and HDL-C decreased from baseline in the probiotics group, but not to a significant degree. This may be at least in part due to the small sample size of our study, as a meta-analysis of 13 trials including 485 patients with high, borderline high and normal cholesterol levels did demonstrate such a difference [23]. Most patients cannot control their cholesterol levels by diet alone. The use of two probiotics in patients with elevated cholesterol resulted in significant decrease in total cholesterol. This treatment also showed cholesterol lowering effects on LDL-C, which is the main component of total cholesterol.
Lactobacillus and Bifidobacterium are lactic acid bacteria which express bile salt hydrolase (BSH). BSH has been suggested to lower serum cholesterol through interactions with host bile salt metabolism [25]. A potential mechanism for the cholesterol lowering effect of probiotics is cholesterol uptake by growing cells, transfer of intracellular cholesterol to the cellular surface, incorporation of cholesterol into the cellular membrane, and coprecipitation of cholesterol with deconjugated bile salt hydrolysate (BSH) [7]. Future studies can explore this mechanism of action.
It is not surprising that patient weight, BMI, and waist and hip circumference of both study groups did not change significantly over the short course of the study. The improvement of systolic blood pressure in the probiotics group is noteworthy, although there was no difference in the two treatment group’s systolic and diastolic blood pressure. A large meta-analysis showed that probiotic fermented milk had significant systolic and diastolic blood pressure lowering effects in both pre-hypertensive and hypertensive subjects [26]. Studies of longer duration with sufficient numbers of subjects are needed to better examine the effect of probiotics on blood pressure.
Study flow chart and enrolment
Baseline demographicsof the study population*
| Probiotics (n=31) | Control (n=33) | p-value |
|---|
| Male, n (%) | 12 (38.7) | 10 (30.3) | 0.60 |
| Age, yr | 49.2 ±(9.1) | 49.± (6.2) | 0.96 |
| Weight, kg | 64.7 ±(11.2) | 69.3± (7.6) | 0.06 |
| Height, cm | 159.1± (7.4) | 161.1 ±(6.1) | 0.25 |
| Waist, cm | 90.0 ±(9.8) | 87.9± (5.3) | 0.29 |
| Hip, cm | 99.3 ±(8.4) | 102.7± (5.3) | 0.05 |
| Body mass index, kg/m2 | 25.6 ±(4.5) | 26.8± (3.3) | 0.25 |
| Systolic blood pressure, mmHg | 139.5± (24.4) | 130.8± (0.07) | 0.07 |
| Diastolic blood pressure, mmHg | 87.7 ±(16.5) | 82.7 ±(8.8) | 0.13 |
*presented as mean (SD)
Serum lipid and fasting blood glucose levels during the study
| Probiotics (n=31) | Control (n=33) | Difference levels from control |
|---|
| Mean (SD) | Mean difference (95% CI) from baseline | Mean (SD) | Mean difference (95% CI) from baseline | Mean difference (95% CI) | p-value |
|---|
| Total Cholesterol, mg/dL |
| Baseline | 237.2 (31.7) | | 231.0 (25.2) | | 6.2 (-8.0 to 20.9) | 0.39 |
| Week 6 | 212.7 (41.4)* | -24.5 (-34.7 to -14.4) † | 252.8 (34.2) | +21.8 (9.4 to 34.2)† | -40.1 (-59.0 to -21.2)* | <0.001 |
| HDL-cholesterol, mg/dL |
| Baseline | 56.5 (16.5) | | 60.7 (18.0) | | -4.2 (-12.9 to 4.4) | 0.33 |
| Week 6 | 52.0 (10.2)* | -4.5 (-9.5 to 0.6) | 59.1 (16.0) | -1.6 (-8.2 to 4.9) | -7.1 (-13.9 to -0.3)* | 0.04 |
| LDL-cholesterol, mg/dL |
| Baseline | | 158.1 (38.8) | | 159.4 (26.7) | -1.4 (-17.9 to 15.2) | 0.87 |
| Week 6 | 153.9 (44.3)* | -4.2 (-16.1 to 7.7) | 182.1 (39.3) | +22.6 (9.4 to 35.0) † | -28.2 (-48.9 to -7.5)* | <0.01 |
| Triglycerides, mg/dL |
| Baseline | | 175.9 (148.2) | | 151.0 (52.4) | +24.9 (-32.5 to 82.3) | 0.38 |
| Week 6 | 148.0 (97.4) | -28.0 (-63.9 to 8.0) | 145 (55.6) | -5.4 (-30.1 to 19.3) | +2.3 (-37.0 to (41.6) | 0.91 |
| Fasting blood glucose, mg/dL‡ |
| Baseline | | 100.0 (91.0-119.0)* | | | 95.0 (90.0-101.0) | 0.04 |
| Week 6 | 97.0 (93.0-108.0) | -2.0 (-11.0 to 9.0) § | 95.0 (91-101) | +2.0 (-5.0 to 6.0) § | | 0.25 |
*Significant difference from Control group (p-value<0.05)
†Significant difference from baseline within each group (p-value<0.05)
‡Presented as median (IQR)
§Median difference (95% CI) from baseline
Limitations
This study had some limitations. An improvement in TC levels was seen in probiotics patients. The short duration of treatment and single time endpoint did not allow determination of the lowest TC achievable or maximum effect with this treatment. Also, patients treated in this short term study were not placed on a controlled or monitored diet. Patients in both groups were advised to minimize their fat consumption, but we did not prescribe a specific amount of fat to be consumed. Further study of combined therapy of probiotics and diet modification is advocated to clarify the optimal efficacy of cholesterol-lowering treatment.The finding that the TC and LDL-C levels of the control group increased at the end of study may be explained by the lack of diet control in the study groups. Finally, the study was limited in duration, not allowing a good assessment of the effect of treatment on secondary endpoints.
Conclusion
A combination of Lactobacillus acidophilus and Bifidobacterium bifidum decreased serum total cholesterol, LDL-cholesterol and HDL-cholesterol levels in hypercholesterolemic patients. There was no effect on serum triglyceride or fasting blood glucose levels. An improvement in systolic blood pressure was seen in this short study. Longer term studies will allow better evaluation of primary and secondary endpoints, including maximum cholesterol lowering effect and blood pressure improvements. A combination of Lactobacillus acidophilus and Bifidobacterium bifidum in capsule form has the potential to lower cholesterol in hypercholesterolemic patients not treated with conventional lipid-lowering drugs.
Acknowledgement
This study was supported by grants from the Faculty of Medicine, Srinakharinwirot University, Thailand.
*presented as mean (SD)
*Significant difference from Control group (p-value<0.05)
†Significant difference from baseline within each group (p-value<0.05)
‡Presented as median (IQR)
§Median difference (95% CI) from baseline
[1]. B Jug, J Papazian, R Lee, MJ Budoff, Association of lipoprotein subfractions and coronary artery calcium in patient at intermediate cardiovascular riskAm J Cardiol 2013 111:213-18. [Google Scholar]
[2]. TJ Anderson, J Gregoire, RA Hegele, 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adultCan J Cardiol 2013 29:151-67. [Google Scholar]
[3]. NJ Stone, JG Robinson, AH Lichtenstein, 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation 2014 129:S1-45. [Google Scholar]
[4]. S Hsu, VK Ton, M Dominique Ashen, A clinician’s guide to the ABCs of cardiovascular disease prevention: the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease and American College of Cardiology Cardiosource Approach to the Million Hearts InitiativeClin Cardiol 2013 36:383-93. [Google Scholar]
[5]. U.S. Food and Drug Administration. FDA Expands Advice on Statin Risks. Available at: http://www.fda.gov/forconsumers/consumerupdates/ucm293330.htm [accessed 02.05.13] [Google Scholar]
[6]. M Bermudez-Brito, J Plaza-Diaz, S Munoz-Quezada, C Gomez-Llorente, A Gil, Probiotic mechanisms of actionAnn Nutr Metab 2012 61:160-74. [Google Scholar]
[7]. M Kumar, R Nagpal, R Kumar, Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseasesExp Diabetes Res 2012 2012:902-17. [Google Scholar]
[8]. IA Abd El-Gawada, EM El-Sayeda, SA Hafezb, HM El-Zeinia, FA Salehb, The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifidobacteria in rats fed on a cholesterol-enriched dietInt Diary J 2005 15:37-44. [Google Scholar]
[9]. AS Akalin, S Gonc, S Duzel, Influence of yogurt and acidophilus yogurt on serum cholesterol levels in miceJ Dairy Sci 1997 80:2721-25. [Google Scholar]
[10]. KK Grunewald, Serum Cholesterol Levels in Rats Fed Skim Milk Fermented by Lactobacillus AcidophilusJ Food Sci 1982 47:2078-79. [Google Scholar]
[11]. MP Taranto, M Medici, G Perdigon, AP Ruiz Holgado, GF Valdez, Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic miceJ Dairy Sci 1998 81:2336-40. [Google Scholar]
[12]. JZ Xiao, S Kondo, N Takahashi, Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteersJ Dairy Sci 2003 86:2452-61. [Google Scholar]
[13]. NM de Roos, G Schouten, MB Katan, Yoghurt enriched with Lactobacillus acidophilus does not lower blood lipids in healthy men and women with normal to borderline high serum cholesterol levelsEur J Clin Nutr 1999 53:277-80. [Google Scholar]
[14]. FD Gilliland, R Mahler, WC Hunt, SM Davis, Preventive health care among rural American Indians in New MexicoPrev Med 1999 28:194-202. [Google Scholar]
[15]. K Hatakka, M Mutanen, R Holma, M Saxelin, R Korpela, Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp shermanii JS administered in capsules is ineffective in lowering serum lipidsJ Am Coll Nutr 2008 27:441-47. [Google Scholar]
[16]. ML Jones, CJ Martoni, M Parent, S Prakash, Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adultsBr J Nutr 2011 107:1505-13. [Google Scholar]
[17]. A Klein, U Friedrich, H Vogelsang, G Jahreis, Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adultsEur J Clin Nutr 2008 62:584-93. [Google Scholar]
[18]. SY Lin, JW Ayres, W Winkler, WE Sandine, Lactobacillus effects on cholesterol: in vitro and in vivo resultsJ Dairy Sci 1989 72:2885-99. [Google Scholar]
[19]. GV Mann, A factor in yogurt which lowers cholesteremia in manAtherosclerosis 1977 26:335-40. [Google Scholar]
[20]. JC Mohan, R Arora, M Khalilullah, Preliminary observations on effect of Lactobacillus sporogenes on serum lipid levels in hypercholesterolemic patientsIndian J Med Res 1990 92:431-32. [Google Scholar]
[21]. M Agerbaek, LU Gerdes, B Richelsen, Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged menEur J Clin Nutr 1995 49:346-52. [Google Scholar]
[22]. RC Weisell, Body mass index as an indicator of obesityAsia Pac J Clin Nutr 2002 11:S681-84. [Google Scholar]
[23]. Z Guo, XM Liu, QX Zhang, Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trialsNutr Metab Cardiovasc Dis 2011 21:844-50. [Google Scholar]
[24]. A Ataie-Jafari, B Larijani, H Alavi Majd, F Tahbaz, Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjectsAnn Nutr Metab 2009 54:22-27. [Google Scholar]
[25]. I De Smet, P De Boever, W Verstraete, Cholesterol lowering in pigs through enhanced bacterial bile salt hydrolase activityBr J Nutr 1998 79:185-94. [Google Scholar]
[26]. JY Dong, IM Szeto, K Makinen, Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trialsBr J Nutr 2013 110:1188-94. [Google Scholar]