Periodontal therapy today has seen a sea of change. New therapies, advanced diagnostic techniques, including a careful analysis of the surrounding tissues as well as strict consideration of biologic principles, now basically characterize Periodontics. This generally translates to the high expectations that patients have from periodontal therapy. Soft tissue aesthetics in dentistry has now taken centre stage.
The dimensions of gingiva and different parts of the masticatory mucosa have become the subject of considerable interest in Periodontics, especially from an aesthetic and therapeutic perspective. The term ‘gingival biotype’ has been used to describe the thickness of gingiva in the facio palatal dimension [1]. Periodontal biotype is considered to be thin- scalloped or thick-flat. Thick, flat gingiva responds to irritation with enlargement and thin and delicate keratinized tissue may result in gingival recession [2]. A direct correlation has been established between gingival biotype and the susceptibility to gingival recession following surgical and restorative procedures [3]. Moreover, gingival thickness plays an important role in wound healing and flap management during regenerative surgical procedures [4]. To predict postoperative outcomes, an accurate pre operative diagnosis of the dimensions of the periodontium becomes necessary. Distinct characteristics of facial gingiva might also be mirrored in the palatal mucosa. Therefore, an accurate diagnosis of gingival biotype, both facial and palatal, is of utmost importance in devising an appropriate treatment plan and achieving a predictable aesthetic and functional outcome.
Although, the thickness of the facial gingiva has been a subject of research since many years, the thickness of the palatal mucosa, in particular, has recently attracted considerable attention with respect to a possible donor site for connective tissue transplants in plastic surgery and for other extra oral applications. Oral connective tissue grafts from the palate are widely used for root coverage procedures, to compensate for defects of the alveolar ridge following tooth extraction and to enhance soft tissue architecture around oral implants [5–7]. Soft tissue grafts harvested from the hard palate have also been applied extraorally for eyelid regeneration, restoration of orbital defects and repair of lip defects [8–10]. Preoperative assessment of several parameters like palatal vault morphology, anatomy of the palatal neurovascular bundle, height, length and the thickness of the palatal tissue is extremely imperative before harvesting palatal connective tissue grafts. The stability of the osseous crest and soft tissue is directly proportional to the thickness of the bone and the gingival tissue. Thick bony plates are associated with thick biotypes and thin plates with potential fenestrations and dehiscence are associated with thin biotypes. Both respond differently to extractions and have a different pattern of osseous remodeling. The remodeling process that follows socket healing, will result in more dramatic alveolar resorption associated with thin biotypes [11,12]. This highlights the importance of appreciating palatal tissue biotypes not only during implant planning in missing teeth, but also during extractions, so as to provide an appropriate implant solution to the patient after extractions.
Moreover, the scanty data available on the dimensions of palatal mucosal thickness in the literature has been obtained in a majority of times, from the Caucasian population. A very few published studies have been carried out which determined the thickness of the palatal gingiva in the Indian population. These studies utilized the conventional method of transgingival probing for the palatal thickness determination. To the best of our knowledge, to date, there is no published study which used an ultrasound device to determine the thickness of the palatal mucosa in the Indian population. This prompted us to undertake this project and study this unexplored area, to some extent.
In view of the above facts, this study was designed to determine and evaluate the variation in the palatal gingival thickness in the Indian population by using an ultrasound device.
Materials and Methods
The subjects were selected from patients visiting the Department of Periodontology, Maratha Mandal’s Nathajirao Guruanna Halgekar Institute of Dental Sciences and Research Centre, Belgaum, Karnataka, India
A total of 50 periodontally healthy subjects (25 males; 25 females) with an age range of 18-23 years were included in the study. The subjects had healthy gingiva with no evidence of bleeding on probing, suppuration or any other clinical signs of inflammation. Subjects with probing depth ≤ 3 mm and with intact interdental papillae in the maxillary anteriors, premolars and molars were included. The teeth examined were devoid of caries or restorations. Subjects having crowding or spacing in maxillary teeth were excluded. Teeth with attrition, abrasion, erosion, subjects taking any medications, subjects undergoing orthodontic treatment or who underwent orthodontic treatment in the past, medically compromised patients, patients having cardiac pacemakers, pregnant women and lactating mothers were excluded from the study. Written informed consents were procured from the subjects prior to the procedure. The study was approved by the Ethical committee of the institution.
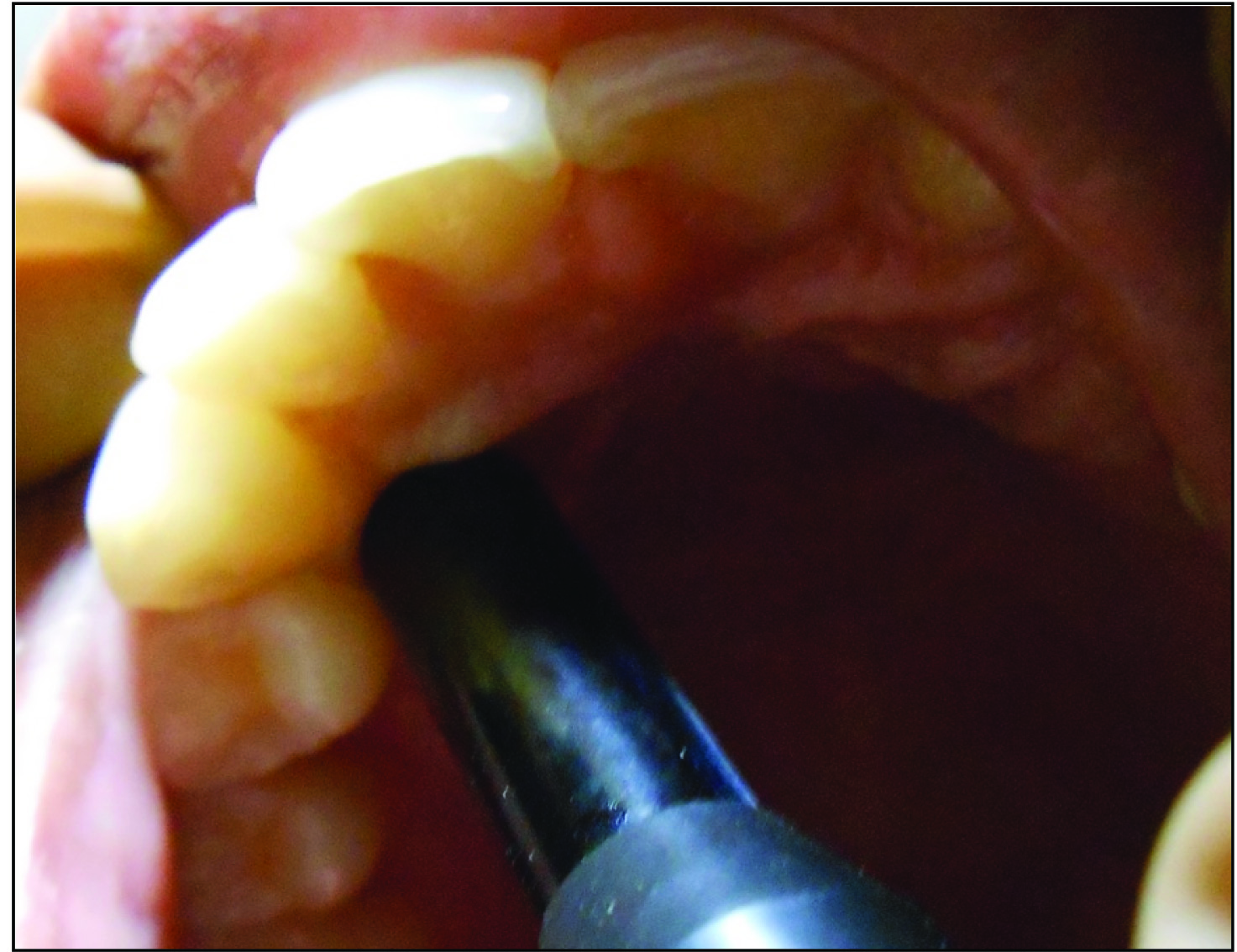
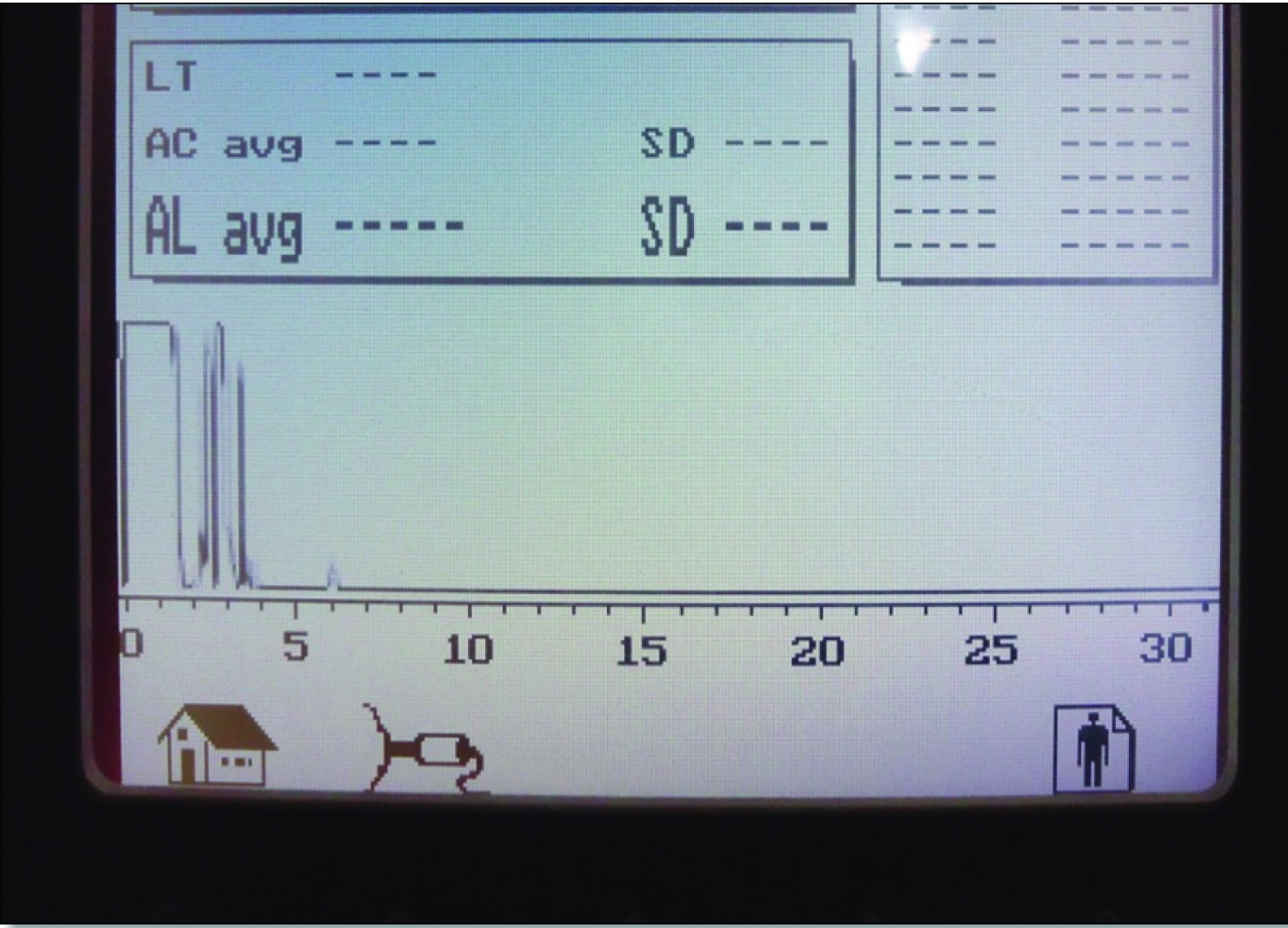
The palatal gingival thickness measurements for the maxillary anteriors, premolars and molars were obtained using the device BIOMETRIC A-SCAN® (Biomedix Optotechnik Pvt Ltd, Bangalore). A sensitive, thin probe attached to the device measured the thickness ultrasonically. The device included a digital display, scan display and a transducer probe [Table/Fig-1,2]. The frequency of the device was 10 MHz and used the pulse echo principle [13]. The transducer probe of the device was adapted in a contact mode, perpendicular to the palatal mucosa at the level of the probing depth and at the mid palatal region of the teeth examined [Table/Fig-3]. The mechanism of action of ultrasound is based on the transit time for the pulse (ultra sound wave) to travel to the bone (hard tissue) and the echo back creates spikes on the digital monitor of the A-scan machine immediately [14]. Utilizing the measurements of the spikes on the graph, the thickness of the palatal gingiva was determined. The digital graph consisted of inbuilt grids, spaced evenly, with a calibration of 0.1 mm. The distance from the end of the reference spike (starting of the measurement spike) to the end of the measurement spike was calculated on the graph according to the grids and the linear scale on the graph [Table/Fig-4].These graphical measurements corresponded to the palatal gingival thickness for the tooth in question.
Ultrasound machine ‘Biometric A-Scan’ that was used for measuring the palatal gingival thickness

Transducer probe attached to the A-scan machine and the red dot depicts the effective beam

The transducer probe adapted in a contact mode on to the palatal gingiva
The ultrasound machine had a digital display on which the graph of ultrasound waves is displayed
Statistical Analysis
The data for palatal gingival thickness at incisors, canines, premolars and molars for the total subjects was represented as Mean ±Standard deviation. The evaluation of variation in the palatal gingival thickness was done by One-way ANOVA test. Pair wise evaluation of the variation in the mucosal thickness was done by Newman-Keuls multiple post hoc procedure. In all the statistical tests employed, p-value ≤ 0.05 was considered statistically significant.
Results
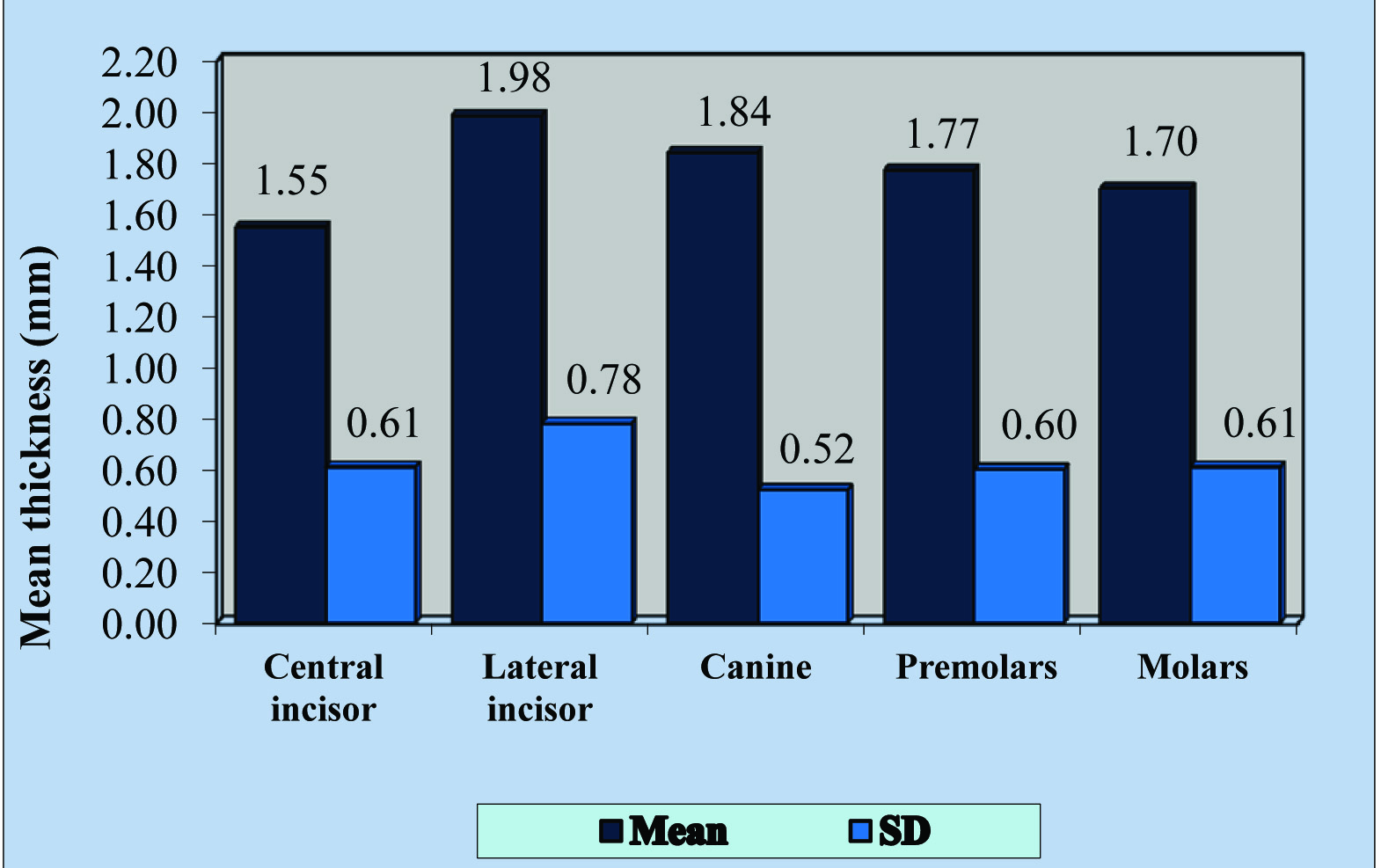
[Table/Fig-5,6] represent the thickness of the palatal mucosa, tooth wise, in all the 50 subjects, in form of Mean and Standard deviation. The mean thickness of the palatal mucosa in the maxillary left and the right quadrants at the central incisor, lateral incisor, canine, premolars and molars was 1.55±0.61 mm, 1.98±0.78 mm, 1.84±0.52 mm, 1.77±0.60 mm and 1.70±0.61 mm respectively.
Graph showing the mean palatal gingival thickness of the total 50 subjects assessed
Data showing the palatal gingival thickness in the study subjects
| Palatal gingival biotypes | n* | Mean±SD† | SE◊ | CV§ |
|---|
| Central incisor | 50 | 1.55 mm±0.61 mm | 0.09 | 39.22 |
| Lateral incisor | 50 | 1.98 mm±0.78 mm | 0.11 | 39.44 |
| Canine | 50 | 1.84 mm±0.52 mm | 0.07 | 28.13 |
| Premolars | 50 | 1.77 mm±0.60 mm | 0.08 | 33.76 |
| Molars | 50 | 1.70 mm±0.61 mm | 0.09 | 35.97 |
*Sample size, †Standard deviation, ◊Standard error, §Coefficient of variation
[Table/Fig-7] depicts the variation in the palatal mucosa of the total subjects at the central incisors, lateral incisors, canines, premolars and molars. Thickness of the palatal mucosa was not uniform and statistically significant variation existed within the palatal mucosal thickness.
Assessment of variation in the palatal gingival thickness in the subjects by One way ANOVA test.
| Source of variation | Degrees of freedom | Sum of squares | Mean sum of squares | f-value* | p-value† |
|---|
| Between biotypes | 4 | 5.2544 | 1.3136 | 3.3209 | 0.0113◊ |
| Within biotypes | 245 | 96.9120 | 0.3956 | |
| Total | 249 | 102.1664 | |
*Value for ANOVA, †p = probability value, ◊p≤0.05 indicates a statistically significant value.
[Table/Fig-8] depicts the variation in the palatal mucosal thickness of the subjects, in a pair wise manner. The thickness was the highest for the lateral incisor (1.98±0.78 mm) followed by canine (1.84±0.52 mm), premolars (1.77±0.60 mm) and molars (1.70±0.61 mm).The thickness was the least at the central incisor region (1.55±0.61 mm).Statistically significant difference in the palatal mucosal thickness existed between central and lateral incisor (p=0.0047). A statistically significant variation in the palatal mucosal thickness was also found at the central incisor and canine (p=0.0495).
Assessment of pair wise variation in the palatal gingival thickness in the subjects by Newman-Keuls multiple post hoc procedure.
| Palatal gingival biotypes | Central incisor | Lateral incisor | Canine | 1st Premolars | 2nd Molars |
|---|
| Mean | 1.55 mm | 1.98 mm | 1.84 mm | 1.77 mm | 1.70 mm |
| Standard deviation (SD) | 0.61 mm | 0.78 mm | 0.52 mm | 0.60 mm | 0.61 mm |
| Central incisor | - | | | | |
| Lateral incisor | *p=0.0047† | - | | | |
| Canine | p=0.0495† | p=0.2442 | - | | |
| Premolars | p=0.1917 | p=0.1908 | p=0.5660 | - | |
| Molars | p=0.2257 | p=0.1067 | p=0.5149 | p=0.5998 | - |
*p = probability value, †p≤0.05 indicates a statistically significant value
Discussion
Contemporary dental therapy is influenced by aesthetics and includes the amalgamation of form and function. To achieve a successful aesthetic outcome in periodontal therapy, dental implants, and restorative procedures a thorough understanding of how tissue responds to therapy is of critical importance. Consequently dental professionals must be fully aware of the soft tissue morphology. The assessment of the thickness of keratinized gingiva, the mucosa of the hard palate and alveolar mucosa is a standard practice in dentistry which aids in a better pre operative diagnosis and treatment planning. Implant planning, restorative dentistry, prosthetic rehabilitation and surgical therapies in the maxillofacial region require an in depth understanding of palatal gingival biotype. Taking into account the usefulness of the palatal mucosa in periodontal plastic procedures and implant therapy, it is quite evident that this tissue holds a prominent position in the current era of soft tissue aesthetics in dentistry.
In the past, most of the studies assessed the palatal mucosa in edentulous subjects who were dentures wearers [15,16]. Very few studies have been conducted which measure the thickness of the palatal mucosa in dentate individuals. The thickness of palatal mucosa has been evaluated by various methods like radiography [17,18], conventional histology on cadaver jaws [19] and invasive methods like transgingival probing by an injection needle or probe [20–23]. Several studies conducted have used non invasive methods like ultrasonic devices of varying frequencies to evaluate the thickness of facial and palatal gingival [24–29]. More recently, computerized tomography has also been used to measure the thickness of palatal mucosa [30,31]. Among all these techniques mentioned above, transgingival probing and the ultrasound methods are the commonly used methods and the results obtained by both the methods are considered to be reliable and accurate [32]. However, we chose to use the ultrasound method as a matter of preference, as this method is simple, quick, non invasive, provides greater patient comfort, has a digital set up for procuring measurements, provides an in built standardisation system for placement of the transducer probe, does not require the fabrication of acrylic stents, does not produce ionizing radiation and does not require the administration of local anesthesia. Moreover, till date, there is no published study which used an ultrasound device to determine the thickness of the palatal mucosa in the Indian population.
The results of the present study show that the palatal gingival thickness among the 50 subjects assessed ranged from with a mean of 1.77 mm. Moreover, the thickness of the palatal gingiva varied at the different teeth sites. The palatal gingiva was the thickest at the lateral incisor region (1.98 mm) followed by the canine region (1.84 mm). The thickness at the premolar and molar region, on an average, was 1.77 mm and 1.7 mm respectively. The thickness was least at the central incisor region (1.55 mm). These variations observed in the subjects at different teeth sites were statistically significant. Hence, it can be inferred that the thickness of the palatal gingiva at different teeth sites was not uniform throughout in the same individual.
Our results are by and large in line with recordings made with other ultrasonic measurement devices employing different frequencies. Uchida et al., [25] demonstrated the palatal thickness by using a B-mode ultra sound device and found the thickness of the palatal mucosa to be ranging from 1.92-2.38 mm.
There are quite a few studies which obtained different measurements compared to our study. Kydd et al., [24] utilized the A mode for measuring the palatal gingival thickness and found it in the range of 2.2 -2.8 mm. Eger et al., [26] assessed the thickness of the palatal mucosa by using an ultrasound device called SDM Krupp with a frequency of 5 MHz and found the thickness in the range of 2.2 -2.9 mm. Waraswapati et al., [20] reported a thicker palatal mucosa ranging from 3–3.5 mm. These differences in the measurements may be attributed to the racial variations, different age groups used and the different methodologies used in the studies. The various frequencies of the different modes of ultrasound devices used, may also contribute to the discrepancies in the measurements.
To the best of our knowledge, very few published studies are available which determined the thickness of the palatal gingiva in the Indian population. Moreover, no study has determined the thickness of palatal gingiva in the Indian population with the use of an ultrasound device. Kolliyavar B et al., [21] used the bone sounding method using a blunt periodontal probe to assess the palatal gingival thickness in canines, premolars and molar regions. Kuriakose A and Raju S [22] used UNC 15 probe with a rubber stopper to assess the thickness of palatal gingiva by bone sounding method. Both the studies, reported a mean gingival thickness ranging from 2.1-3.5 mm and 2.1–3 mm respectively, which was greater than the results obtained in our study. This could be attributed to the tissue edema produced by the local anesthesia administered prior to the bone sounding technique. In our study, the ultrasound device was used in the non compression, contact mode and did not require the administration of local anesthesia. The readings hence obtained, could be considered as tangible and accurate.
Biometric A-Scan, which is an ultrasound device, was able to determine the palatal gingival thickness atraumatically and painlessly in contrast to the conventional methods of transgingival probing. Transgingival probing is rather an invasive method and may give inaccurate measurements because of the tissue edema occurring due to injection of local anesthesia prior to the procedure [33]. Moreover, while using transgingival probing with blunt calibrated periodontal probes or endodontic reamers, the measurements are rounded off to the nearest millimeter. Apart from this, blunt periodontal probes may underestimate the thickness by partially penetrating the tissues, while sharp reamers may overestimate the thickness by penetrating deeper into areas where porous bone is present. In contrast to this, the grids obtained on the digital graph of the A Scan device are spaced 0.1 mm apart, which offers precise measurements of the gingival tissues. The sharp spikes are created on the digital display at the level of the reference spike only when the transducer probe is kept perpendicular to the palatal mucosa. Hence, as an advantage, the A Scan device offers an in built standardization system by giving a guide to accurately place the probe, displayed by the amplitude of the spikes. It can be thus said, that the ultrasound device is an elegant means of obtaining gingival thickness non-invasively, accurately and rapidly and does not require any administration of local anesthesia.
Limitations
The transducer probe of the ultrasound has limited access to the posterior most areas of oral cavity. The ultrasound method is technique sensitive and is not as cost effective as transgingival probing. Thickness of palatal gingiva may be influenced by other factors such as genetics, body mass, geographic locations, racial differences, anatomy of the palatal area, location of the palatal neurovascular bundle, keratinization and the influence of rugae patterns. Hence, a more extensive research is required to support this hypothesis. More data needs to be collected and evaluated to establish substantial evidence.
Conclusion
Our endeavour in this research project attempts to open more avenues for studies in the field of advanced periodontal diagnosis and expand the horizons of treatment planning in periodontal plastic surgeries and implant therapy. In planning periodontal plastic procedures, in situations wherein the canine-premolar area does not yield an adequate thickness, we suggest the inclusion of lateral incisor area as a potential donor site for graft harvesting. In this regard, ultrasonographic measurements provide an elegant means of obtaining the measurements of gingival and mucosal tissues rapidly, accurately and non-invasively.
*Sample size, †Standard deviation, ◊Standard error, §Coefficient of variation
*Value for ANOVA, †p = probability value, ◊p≤0.05 indicates a statistically significant value.
*p = probability value, †p≤0.05 indicates a statistically significant value