Longitudinal Telomere Erosion in Lymphocyte Subsets of Patients with Atherosclerotic Peripheral Arterial Disease (PAD)
Dirk de Beer1, Jan Völzmann2, Christoph Kalka3, Gabriela M. Baerlocher4
1Experimental Hematology, Department of Clinical Research, Department of Hematology, University of Bern, Bern Switzerland; University Hospital/Inselspital Bern, Bern, Switzerland.
2Department of Clinical and Interventional Angiology, University Hospital Bern, Bern.
3Department of Clinical and Interventional Angiology, University Hospital Bern, Bern.
4 Experimental Hematology, Department of Clinical Research; Department of Hematology,University of Bern, Bern Switzerland; , University Hospital/Inselspital Bern, Bern, Switzerland.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dirk de Beer, University Hospital and University of Bern, Experimental Hematology, Department of Clinical Research, Freiburgstrasse 4, CH-3010, Bern.
E-mail: dirk_de_Beer@t-online.de
Telomere attrition has been linked to accelerate vascular ageing and seems to predispose for vascular disease. Our aim was to study the telomere length dynamics over time and in subsets of leukocytes from 15 patients with peripheral arterial disease (PAD).
The mean telomere length in subsets of leukocytes of patients with PAD was in the normal range of age-related telomere length values from healthy individuals. However, we found significant higher telomere attrition for T-cells from patients with PAD over a time period of six months when compared to the controls.
The higher telomere loss in T-cells of patients with PAD most likely reflects a higher cell turnover of this leukocyte subset, which is involved in the process of chronic inflammatory disease underlying vascular disease. Further studies are needed to confirm these data and to assess how far this T-cell telomere attrition will correlate to the extent of the disease.
Arteriosclerosis, CAD, PAD, Telomere length
Introduction
PAD is the most common and important type of peripheral vascular disease (PVD). PAD increases with age, and by the age of 65 years, about 12 to 20 percent of the population has PAD. Diagnosis is critical, as people with PAD have a four to five time’s higher risk of a heart attack or stroke [1]. Besides several well known reversible risk factors like smoking, obesity, high blood pressure or physical inactivity [2]the main irreversible risk factor for PAD is aging.
Telomere length can be regarded as a marker of cellular age since telomeres shorten with cellular replication approximately 50–100bp per cell division. When telomeres become critically short this leads to senescence and apoptosis. Chronic inflammation and oxidative stress are involved in the pathogenetic process of atherosclerosis. Both factors lead to increased hematopoietic cell turnover resulting in telomere shortening [3,4]. Referring to a model stated by Aviv this accelerated telomere erosion may contribute to progressive endothelial dysfunction since the hematopoietic system and the vascular endothelium share a common embryonic origin [5,6]. Shortened telomere length may reflect diminished hematopoietic stem cell (HSC) reserves at birth or their accelerated attrition rate afterward. Indeed, a characteristic senescent phenotype is observed in the endothelium of atherosclerotic lesions [7]. Moreover, it has been shown, that the endothelial function and the inflammatory status are related to the severity of PAD [8].
In contrast to studies describing telomere length in coronary artery disease (CAD), these studies in patients with PAD are completely missing. In this pilot study on patients with PAD we describe our data of telomere length measurements in different subsets of leukocytes (granulocytes, B-cells, naïve and memory T-cells, NK/NKT-cells) and how the telomere length in these subsets changes over time.
Materials and Methods
In this pilot study (duration: 25 month, beginning: October 2009, end: November 2011) 15 patients (median age 66.8, range: 56–74, gender: 10 male, 5 female) with mild to moderate intermittent lengthclaudication, Fontaine stage IIa/b were included. PAD was diagnosed either by angiographic documented disease or by the presence of an ankle brachial index (ABI) <0.9. After informed consent leukocytes were obtained from the peripheral blood of the patients at various time points (0, 3, 6 months) and telomere length measurements were performed in subsets of leukocytes (granulocytes, CD20-positive B-cells, CD45RA-positive naïve T-cells, CD45RA-negative T-cells, CD57-positive NK/NKT-cells) by automated multicolor flow-FISH [9]. Telomere length measurements in subsets of leukocytes of over 380 healthy individuals (age range 0-102 y) served for the calculation of normal reference ranges [10,11]. Five healthy individuals without a history or obvious signs of PAD served as internal controls. Statistical analyses were done by the use of the Excel Analysis ToolPak (Microsoft).
Results
Triglycerides ranged from 0.4mmol/L to 2.0mmol/L (mean: 1.19mmol/L, normal value: <2.3 mmol/l) and the total cholesterol from 3.2mmol/L to 6.3mmol/L (mean: 4.81mmol/L, normal value: <5.1mmol/l) with 12 patients being treated for hyperlipidemia. 14 patients were smokers, 12 patients had a history of hypertension and five were type 2 diabetics. The mean body-mass-index (BMI) was 26.7, ranging from 19.8 to 32.2 (normal: 18.5 to 25 [Table/Fig-1]).
The mean telomere length for the total leukocytes of all 15 patients was in the normal range (length ± STD: 5.46kb ± 0.43kb) and was not significantly different from healthy controls (5.49kb ± 0.45kb, p= 0.91). The telomere length in granulocytes was approximately 200 bp shorter in patients with PAD compared to granulocytes in the control group but this difference reached not statistical significance (5.83kb ± 0.35kb vs. 5.99kb ± 0.73kb p=0.66). The telomere length in lymphocytes was equal in both groups (5.01kb ± 0.41kb vs. 5.00kb ± 0.65, p= 0.97).
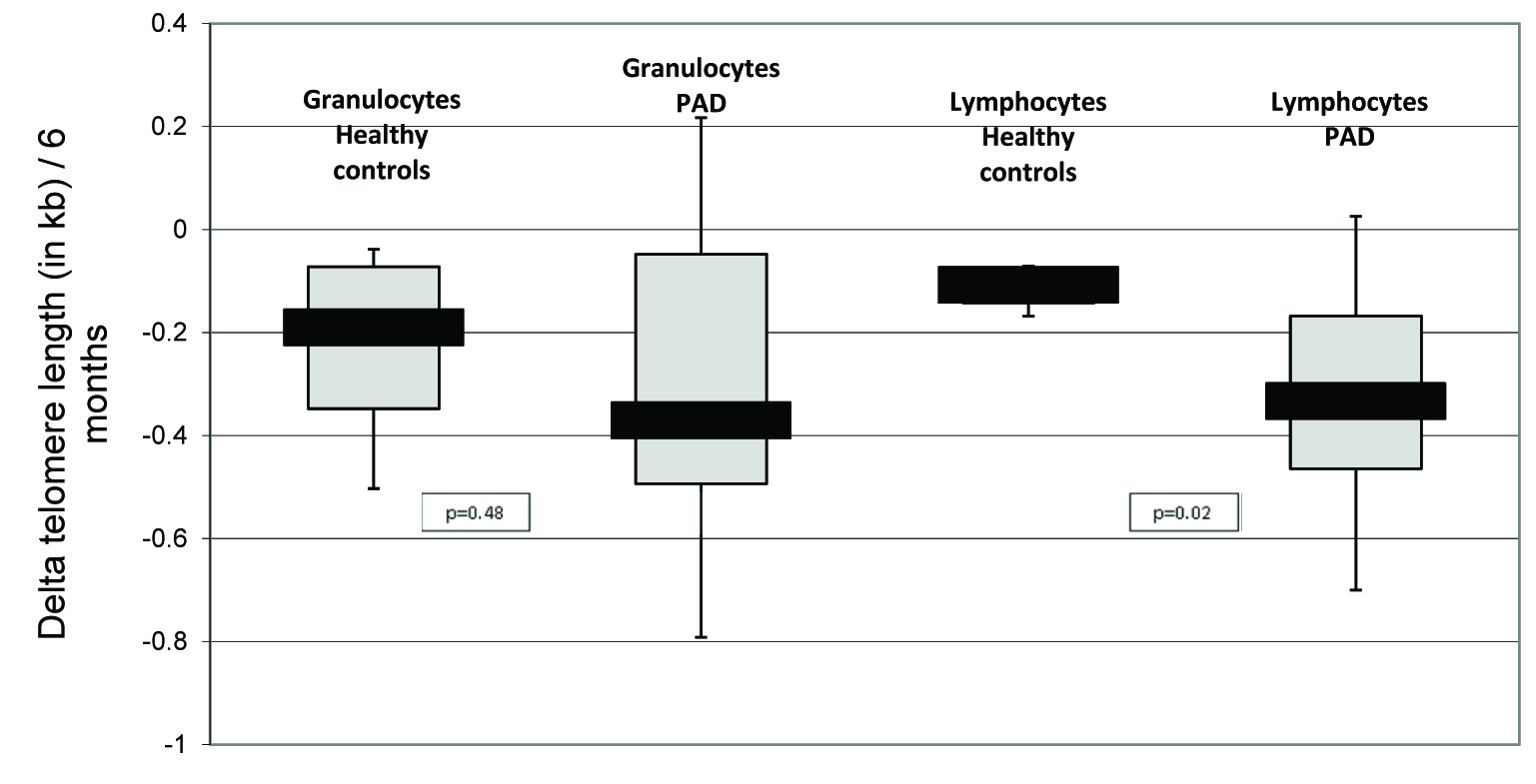
However, when we investigated the telomere length dynamics over a time period of six months we found a significant higher telomere attrition for lymphocytes from patients with PAD (mean ± STD: -0.331kb ± 0.320kb) when compared to the controls (mean ± STD -0.113kb ± 0.046kb, p=0.02, [Table/Fig-2]). Telomere attrition in granulocytes did hardly demonstrate any differences between patients with PAD and healthy controls (mean ± STD for granulocytes: -0.342kb ± 0.412kb vs. -0.230kb ± 0.214kb, p=0.48). To address this difference in telomere attrition in lymphocytes over time more specifically to a certain cell type, we evaluated in a next step the telomere length in B-cells, naïve and memory T-cells and NK/NKT-cells. While the telomere length attrition was significantly accelerated in both types of T-cells (mean ± STD PAD vs. healthy controls: -0.493kb ± 0.371kb vs. -0.168kb ± 0.046kb, p=0.005 for naïve T-cells, -0.507kb ± 0.511kb vs. -0.054kb vs. 0.161kb, p=0.002 for memory T-cells) telomere length attrition did not differ significantly in B-cells and NK/NKT-cells when PAD patients were compared to healthy controls (mean ± STD PAD vs. healthy controls: -0.330kb ± 0.414kb vs. -0.256kb ± 0.156kb, p=0.58 for B-cells, -0.269kb ± 0.716kb vs. -0.323kb ± 0.292kb, p=0.82 for NK/NKT-cells).
Discussion
In contrast to CAD where clearly shortened telomeres can be observed [12] we found no difference in the overall leukocyte telomere length for patients with PAD in comparison to healthy controls. Nevertheless, in T-cells of patients with PAD we found significantly accelerated telomere erosion over a time period of six months. This result reflects an involvement of T-cells in PAD. Furthermore it supports the hypothesis that telomere length erosion is rather a consequence of a higher cell turnover of certain lymphocytes caused by chronic inflammation and oxidative stress than the primary abnormality leading to atherosclerosis. The question whether short telomeres are a cause or the consequence of PAD is much debated [13]. How far the slightly higher telomere attrition over six months in T-cells of patients with PAD will have an impact on the overall telomere length in the future is not yet clear. Nevertheless, if leukocyte telomere attrition starts in T-cells an early screening for telomere shortening in T-cells could be a possibility to identify patients which are on the way to develop a more severe form of the disease. Early intervention or changes in lifestyle could prevent later surgeries.
One should keep in mind that it is not clear how long the disease already existed at the time of telomere length measurement. Telomere lengths in the normal range could be found in patients with early stages of disease. In patients with CAD significant telomere attrition can already be found in early stages of disease [14]. The difference in the telomere length attrition between PAD and CAD may also result from a much lower cellular turnover by the chronic inflammation of PAD compared to a much higher cellular turnover in CAD. In addition, it has been described that telomerase is down-regulated in endothelial progenitor cells in CAD leading to a higher loss of telomere repeats [15], which might not be the case in PAD.
| PAD | Controls |
|---|
| Total (n=34) | Follow-up (n=15) | Total (n=17) | Follow-up (n=5) |
|---|
| Age (mean, range) [years] | 67 (56-74) | 67 (56-74) | 59 (51-71) | 63 (53-71) |
| Gender (no of m/f) | 23/10 | 10/5 | 7/10 | 3/2 |
| No of smokers never/past (stop>10yr)/current | 4/30/0 | 1/14/0 | 9/8/0 | 4/1/0 |
| Hight (mean ± STD) [m] | 1.68 ± 0.07 | 1.71 ± 0.07 | 1.70 ± 0.09 | 1.71 ± 0.13 |
| Weight (mean ± STD) [kg] | 80.2 ± 14.6 | 76.6 ± 14.0 | 74.8 ± 11.3 | 79.2 ± 3.4 |
| Waist circumference (mean ± STD) [cm] | 102.6 ± 15.5 | 100.8 ± 14.1 | 93.9 ± 10.9 | 101.0 ± 7.1 |
| Hip circumference (mean ± STD) [cm] | 105.7 ± 9.8 | 104.1 ± 9.2 | 103.6 ± 8.6 | 107.8 ± 8.2 |
| Waist to hip ratio (mean ± STD) | 0.97 ± 0.09 | 0.96 ± 0.01 | 0.91 ± 0.07 | 0.94 ± 0.05 |
| BMI (mean ± STD) [kg/m2) | 28.4 ± 5.2 | 26.7 ± 3.8 | 25.8 ± 3.6 | 27.3 ± 4.0 |
| Diabetes (y/n) | 15/19 | 5/10 | 0/17 | 0/5 |
| Total cholesterol<5 (mmol/l) | 4.6 ± 0.9 | 4.81 ± 0.69 | 5.6 ± 1.4 | 5.50 ± 0.97 |
| HDL cholesterol>1 (mmol/l) | 1.6 ± 0.4 | 1.81 ± 0.44 | 1.6 ± 0.3 | 1.55 ± 0.33 |
| LDL cholesterol<2.6 (mmol/l) | 2.4 ± 0.6 | 2.5 ± 0.6 | 3.0 ± 0.8 | 3.4 ± 0.8 |
| Triglycerides< 1.71 (mmol/l) | 1.4 ± 0.6 | 1.19 ± 0.52 | 2.1 ± 3.3 | 1.11 ± 0.42 |
| ABI (0.9-1.2) mmHg | 0.8 ± 0.2 | 0.80 ± 0.12 | 1.2 ± 0.1 | 1.18 ± 0.09 |
| No of pat with hypertension (>140/90mmHg) [y/n] | 30/4 | 12/3 | 5/12 | 2/3 |
| No of pat with hyperlipidemia (y/n)* | 31/3 | 12/3 | 14/3 | 3/2 |
*According to classification by Fredrickson
Slightly increased telomere attrition over 6 months in lymphocytes of patients with PAD compared to healthy controls
Boxplot of the telomere attrition in granulocytes and lymphocytes derived from peripheral blood of patients with PAD and healthy controls. Telomere attrition has been calculated as difference between the initial measurement and the follow-up measurement 6 months later. Black bars indicate the median (50%), grey bars respectively the higher (75%) or the lower quartiles (25%)
Conclusion
Since we found in patients with PAD telomere attrition, but not significantly shortened telomeres, we assume that telomere attrition in PAD is in contrast to CAD a cause of the disease but not a precondition for the development. Therefore, this study points to a different pathophysiology in patients with PAD and CAD. Early monitoring of telomere length changes over time could be a helpful tool for early recognition of patients developing PAD which should be examined by continuing studies.
*According to classification by Fredrickson
[1]. MH Criqui, Peripheral arterial disease--epidemiological aspectsVasc Med 2001 6(3 Suppl):3-7. [Google Scholar]
[2]. CD Lewis, Peripheral arterial disease of the lower extremityJ Cardiovasc Nurs 2001 15(4):45-63. [Google Scholar]
[3]. G Voghel, N Thorin-Trescases, N Farhat, A Nguyen, L Villeneuve, Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factorsMech Ageing Dev 2007 128(11-12):662-71. [Google Scholar]
[4]. R Rossn, Atherosclerosis--an inflammatory diseaseN Engl J Med 1999 340(2):115-26. [Google Scholar]
[5]. A Aviv, D Levy, Telomeres, atherosclerosis, and the hemothelium: the longer view. Annu Rev Med 2012 63:293-3. [Google Scholar]
[6]. A Aviv, Genetics of leukocyte telomere length and its role in atherosclerosisMutat Res 2012 730(1-2):68-74. [Google Scholar]
[7]. T Minamino, H Miyauchi, T Yoshida, I Komuro, Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunctionCirculation 2002 105(13):1541-44. [Google Scholar]
[8]. A Silvestro, F Scopacasa, A Ruocco, G Oliva, V Schiano, C Zincarelli, Inflammatory status and endothelial function in asymptomatic and symptomatic peripheral arterial diseaseVasc Med 2003 8(4):225-32. [Google Scholar]
[9]. GM Baerlocher, I Vulto, G de Jong, PM Lansdorp, Flow cytometry and FISH to measure the average length of telomeres (flow FISH)Nat Protoc 2006 1(5):2365-76. [Google Scholar]
[10]. BP Alter, GM Baerlocher, SA Savage, SJ Chanock, BB Weksler, JP Willner, JA Peters, Very short telomere length by fluorescence in situ hybridization identifies patients with dyskeratosis congenitaBlood 2007 1(5):1439-47. [Google Scholar]
[11]. G Aubert, GM Baerlocher, I Vulto, SS Poon, PM Lansdorp, Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genesPLoS Genet 2012 8(5):e1002696doi: 10.1371/journal.pgen.1002696 [Google Scholar]
[12]. NJ Samani, R Boultby, R Butler, JR Thompson, AH Goodall, Telomere shortening in atherosclerosisLancet 2001 358(9280):472-73. [Google Scholar]
[13]. SW Brouilette, JS Moore, AD McMahon, JR Thompson, I Ford, J Shepherd, Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control studyLancet 2007 26(9556):107-14. [Google Scholar]
[14]. K Vemarala, A Roy, VK Bahl, D Prabhakaran, N Nath, S Sinha, Early accelerated senescence of circulating endothelial progenitor cells in premature coronary artery diseasepatients in a developing country - a case control studyBMC Cardiovasc Disord 2013 (19):13-104. [Google Scholar]
[15]. M Satoh, Y Ishikawa, Y Takahashi, T Itoh, Y Minami, M Nakamura, Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery diseaseAtherosclerosis 2008 198(2):347-53. [Google Scholar]