Overactive bladder (OAB) is a clinical syndrome defined by symptoms of urinary urgency with or without urge incontinence, and usually with frequency and nocturia [1]. The prevalence of OAB is higher in men (42%) than women (31%) over 75 years of age [2]. The prevalence of OAB in Asia is around 53.1% [3].
Management of OAB involves the practice of lifestyle and behavioral modifications, bladder exercises and pharmacotherapy, either alone or in combination. Antimuscarinic agents, the pharmacotherapy of choice for OAB, include oxybutynin, tolterodine, darifenacin, solifenacin and trospium. Though the above antimuscarinics are similar in their efficacy as observed in various randomized controlled studies, differences in their side effect profiles may exist due to differences in affinity for muscarinic receptor subtypes, organ selectivity and pharmacokinetics [2,4]. Dry mouth and constipation are the most common adverse effects reported in various studies [5]. Constipation can lead to abdominal pain, malnutrition and fecal impaction which may significantly impair the quality of life [5].
The present study intends to prospectively compare the occurrence and severity of constipation with the above two drugs in OAB patients, which would guide the choice of therapy in patients at an increased risk of constipation and its consequences like elderly, patients on drugs with antimuscarinic properties for other indications, patients on aluminum antacids, opioids, calcium channel blockers, history of anorectal surgery and those at risk of urinary or gastric retention.
Materials and Methods
The study was conducted following approval from the institutional ethics committee at Urology OPD, KIMS Hospital and Research Centre, Bangalore, India. Written informed consent was obtained from all the study subjects/legal representatives, after fully explaining the study procedure to their satisfaction. The study was conducted in accordance with international conference on harmonization guidelines on good clinical practice and the Helsinki declaration of 1975, revised in 2000. The study was registered retrospectively with the Clinical Trial Registry, India (CTRI/2014/09/005000).
A total number of 60 subjects were included between November 2013 and June 2014 for the present prospective, comparative, open label, parallel group study. A detailed record of personal history, present and past medical history, drug history, toileting facilities, mobility, and nutritional (fiber) and daily fluid intake was taken. The (Overactive bladder symptom score) OABSS [1] was used to clinically diagnose OAB. It includes four parameters, frequency (0 to 2), nocturia (0 to 3), urgency (0 to 5) and urge urinary incontinence (0 to 5), and the score of each increases with the severity. OABSS was administered at baseline, 2 and 4 weeks of treatment initiation to monitor the treatment response. Relevant investigations were done to detect any underlying complications associated with OAB and also to rule out any contraindications to the study medications.
The selected patients were randomized into two groups of 30 in each, using a randomization table, to receive darifenacin 7.5 mg or trospium 60 mg (extended release preparation). Darifenacin (with or without food) and trospium (one hour before meals with full glass of water) were administered once daily, orally, either in the morning or at bedtime. A wash out period of 14 days was allowed for those subjects previously receiving any antimuscarinics. Patients were evaluated with McMillan & Williams, 1989 Constipation assessment scale (CAS) [11], Bristol stool form scale [12] and The Knowles-Eccersley-Scott-Symptom (KESS) questionnaire score [13] for the presence or absence, severity of constipation and their effect on patient well-being at baseline, 2 weeks and 4 weeks. Each item in the CAS is graded as either no problem (0), some problem (1) or severe problem (2) and the symptoms include abdominal distension or bloating, change in amount of gas passed rectally, less frequent bowel movements, oozing liquid stools, rectal fullness or pressure, rectal pain with bowel movement, small volume of stool and unable to pass stools. The total score is generated by the sum of individual scores. The Bristol stool form scale for consistency of stool has 7 types. Type 1 refers to stool in the form of separate hard lumps or nuts. The consistency decreases with increasing number, with type 7 indicating entirely liquid watery stool with no solid pieces. The 11 item KESS questionnaire includes duration of constipation (0 to 4), duration of laxative use (0 to 3), frequency of bowel movement (0 to 3), number of unsuccessful evacuatory attempts (0 to 3), frequency of feeling of incomplete evacuation (0 to 4), frequency of abdominal pain (0 to 4), severity of bloating (0 to 4), frequency of enemas/digitation (0 to 4), time taken in minutes in lavatory per attempt (0 to 3), frequency of painful evacuation (0 to 4) and stool consistency without laxatives (0 to 3). The total score ranges from 0 to 39, with higher score indicating higher severity of constipation. Both the study medications were continued for 4 weeks. The patients were assessed with the above three subjective constipation assessment questionnaires after 2 and 4 weeks of treatment initiation to evaluate the occurrence and impact of constipation. During the study period, the patients were instructed not to crush, chew or open the capsule/tablet, to establish a regular toileting pattern and position, maintain uniform fluid and fiber intake and to contact the doctor immediately in case of any serious adverse event.
Inclusion Criteria
Male and female patients aged ≥50 and <80 years who were diagnosed with OAB based on their overactive bladder symptoms.
Patients with a baseline score for urinary urgency of ≥2 points, day time frequency >1 point and a total OABSS of ≥3 points, with or without urge urinary incontinence (UUI) episodes.
Stable doses of alpha blockers or 5-alpha-reductase inhibitors for patients with benign prostatic hyperplasia were permitted with the limitation that a specific drug was used without any change in dosage and administration, and was not replaced with another drug during the study period.
Exclusion Criteria
Serious heart disease (New York Heart Association class III or IV)
Untreated angle-closure glaucoma,
Myasthenia gravis,
Gastric outlet or intestinal obstruction, paralytic ileus, gastric and intestinal atony or risk of urinary or gastric retention.
Prostate or bladder cancer,
Urinary tract infection,
Concomitant medications known to affect urinary bladder function like anticholinergics, antispasmodics, antidepressants, first generation antihistaminics, cholinergics, potent inhibitors of cytochrome CYP3A4 (ketoconazole, itraconazole, ritonavir, nelfinavir, clarithromycin and nefazadone), potent P-glycoprotein inhibitors (cyclosporine and verapamil), aluminum antacids, antiparkinson and antipsychotic drugs, calcium channel blockers, opioids, muscle relaxants, angiotensin-converting enzyme inhibitors and anticoagulants.
Severe hepatic dysfunction (Child Pugh score ≥ 10) or severe renal dysfunction (Creatinine clearance < 30ml/ min),
Participation in any clinical trial within 30 days before study entry.
Patients with severe constipation (<3 defecations a week).
History of alcohol or drug abuse,
Currently suffering from serious disease or malignancy
Significant psychiatric problems.
Withdrawal Criteria
Failure of antimuscarinic therapy.
Development of serious adverse event.
Statistical Analysis
Calculation of sample size: The incidence of constipation with trospium and darifenacin as reported in earlier studies is 4.8% and 14.8% respectively [5,8]. For the present parallel group study design, with non-inferiority criteria set at 10%, power at 80% and a drop-out rate at 5%, the total sample size of 56 was arrived at, with 28 in each group. However, 30 were included in each treatment group to further increase the power of the study.
The pre, mid and post-treatment comparisons within each group were performed with Friedman’s test. If the p-value was < 0.05, Wilcoxon signed ranks test was used to compare between the two observations to know the exact point of statistical significance. The differences in symptom scores between groups were compared using Mann–Whitney test with the level of significance set at p < 0.05.
Results
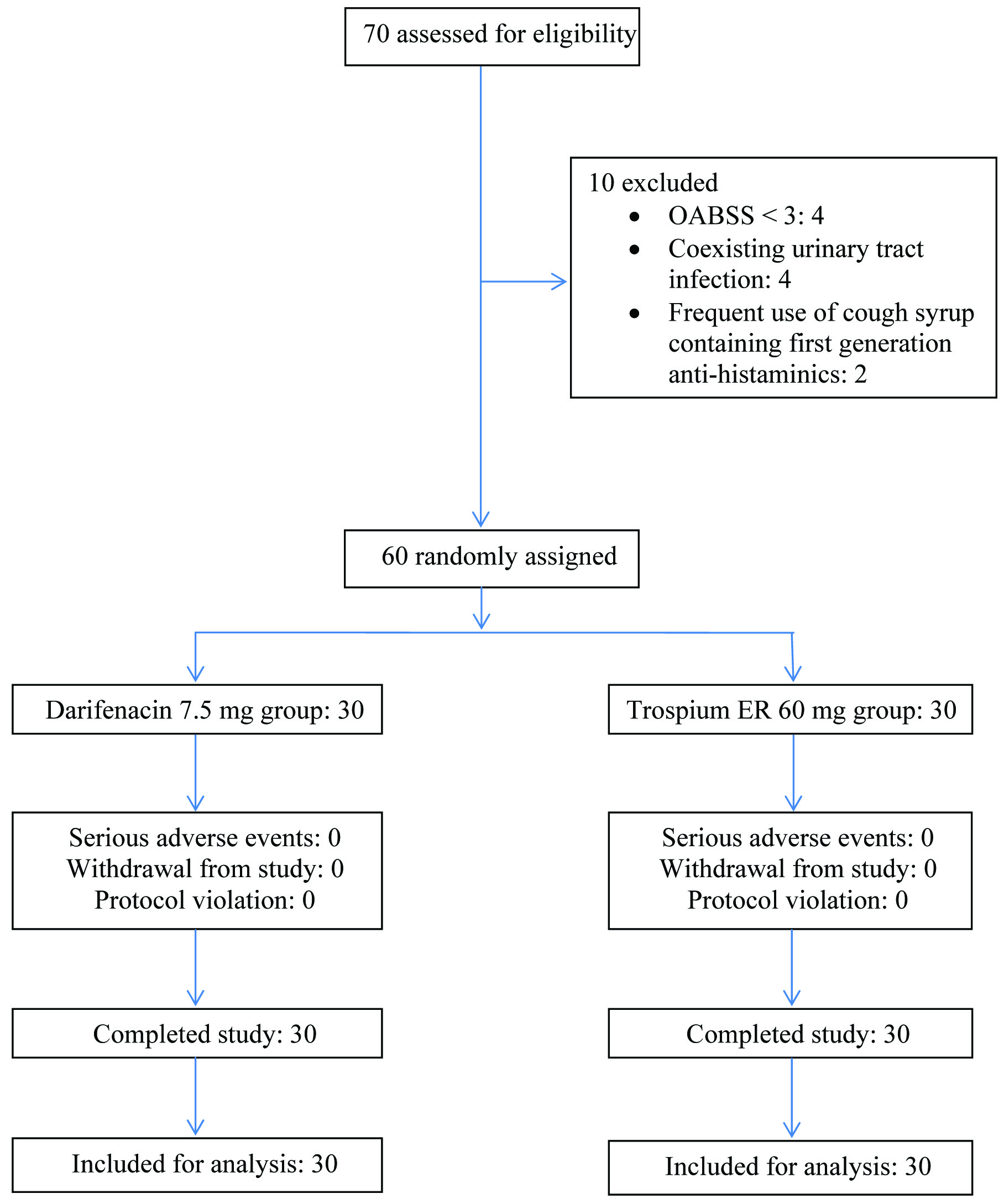
Sixty patients who met the eligibility criteria were randomly assigned to two treatment groups and received darifenacin 7.5mg or trospium 60mg, once daily, orally in 1:1 ratio. None withdrew from the study and there were no protocol violations. All the 30 patients in both the groups completed the study and were included for analysis [Table/Fig-1]. [Table/Fig-2] shows demographic and baseline characteristics of the subjects, which were comparable across the treatment groups. Patients in the darifenacin and trospium groups had a mean age of 63.27 ± 9.15 and 64.87 ± 7.97 years, baseline OABSS of 9.87 ± 3.75 and 9.27 ± 3.21, McMillan and Williams CAS of 0.27 ± 0.58 and 0.53 ± 1.17, KESS questionnaire score of 3.73 ± 3.45 and 3.33 ± 4.41 and Bristol stool consistency of 2.6 ± 0.97 and 3.07 ± 0.87 respectively.
Baseline demographic and clinical characteristics
| Darifenacin (n = 30) | Trospium (n = 30) |
|---|
| Age (years) | Mean ± SD | 63.27 ± 9.15 | 64.87 ± 7.97 |
| Gender (Male) | n (%) | 22 (73.30) | 24 (80.00) |
| Fluid intake / day (ml) | Mean ± SD | 2133.33 ± 706.29 | 2466.67 ± 973.20 |
| Defecations / day | Mean ± SD | 1.07 ± 0.25 | 1.00 ± 0.00 |
| Systolic BP (mm Hg) | Mean ± SD | 146.67 ± 17.06 | 131.47 ± 18.83 |
| Diastolic BP (mm Hg) | Mean ± SD | 90.13 ± 11.49 | 83.33 ± 8.81 |
| Weight (kg) | Mean ± SD | 62.97 ± 18.35 | 70.07 ± 12.66 |
| BMI (kg / m2) | Mean ± SD | 24.21 ± 6.14 | 24.63 ± 4.25 |
| Waist circumference (cm) | Mean ± SD | 91.07 ± 16.34 | 95.53 ± 9.63 |
| OABSS | Mean ± SD | 9.87 ± 3.75 | 9.27 ± 3.21 |
| McMillan and Williams CAS | Mean ± SD | 0.27 ± 0.58 | 0.53 ± 1.17 |
| KESS questionnaire score | Mean ± SD | 3.73 ± 3.45 | 3.33 ± 4.41 |
| Bristol stool consistency | Mean ± SD | 2.6 ± 0.97 | 3.07 ± 0.87 |
At the end of the study period, the change in OABSS was 58.76% and 56.85% in darifenacin and trospium treatment groups respectively. OABSS improved significantly, -5.80 ± 3.99 (p = 0.0005) and -5.27 ± 2.98 (p = 0.0005) in darifenacin and trospium groups respectively [Table/Fig-3]. However, there was no significant difference in improvement between the two groups either at two weeks (p = 0.952) or four weeks (p = 0.654).
Comparison of change in OABSS and its individual subjective symptoms
| Darifenacin | Trospium |
|---|
| Baseline score | 4 week score | Change in score from baseline | % change | Baseline score | 4 week score | Change in score from baseline | % change |
|---|
| Mean ± SD | Mean ± SD |
|---|
| Frequency | 1.00 ± 0.74 | 0.20 ± 0.55 | -0.80 ± 0.76 | 80.00 | 0.53 ± 0.73 | 0.07 ± 0.25 | -0.47 ± 0.63 | 86.79 |
| Nocturia | 2.80 ± 0.85 | 1.93 ± 1.08 | -0.87 ± 0.90 | 31.07 | 2.27 ± 1.01 | 1.33 ± 0.96 | -0.93 ± 0.79 | 41.41 |
| Urgency | 3.40 ± 1.99 | 1.53 ± 2.22 | -1.87 ± 2.22 | 55.00 | 3.87 ± 1.61 | 1.47 ± 2.13 | -2.40 ± 2.19 | 62.02 |
| Urge urinary incontinence | 2.67 ± 1.95 | 0.40 ± 1.04 | -2.27 ± 1.72 | 85.02 | 2.60 ± 2.03 | 1.13 ± 1.78 | -1.47 ± 1.96 | 56.54 |
| OABSS | 9.87 ± 3.75 | 4.07 ± 3.29 | -5.80 ± 3.99 | 58.76 | 9.27 ± 3.21 | 4.00 ± 3.86 | -5.27 ± 2.98 | 56.85 |
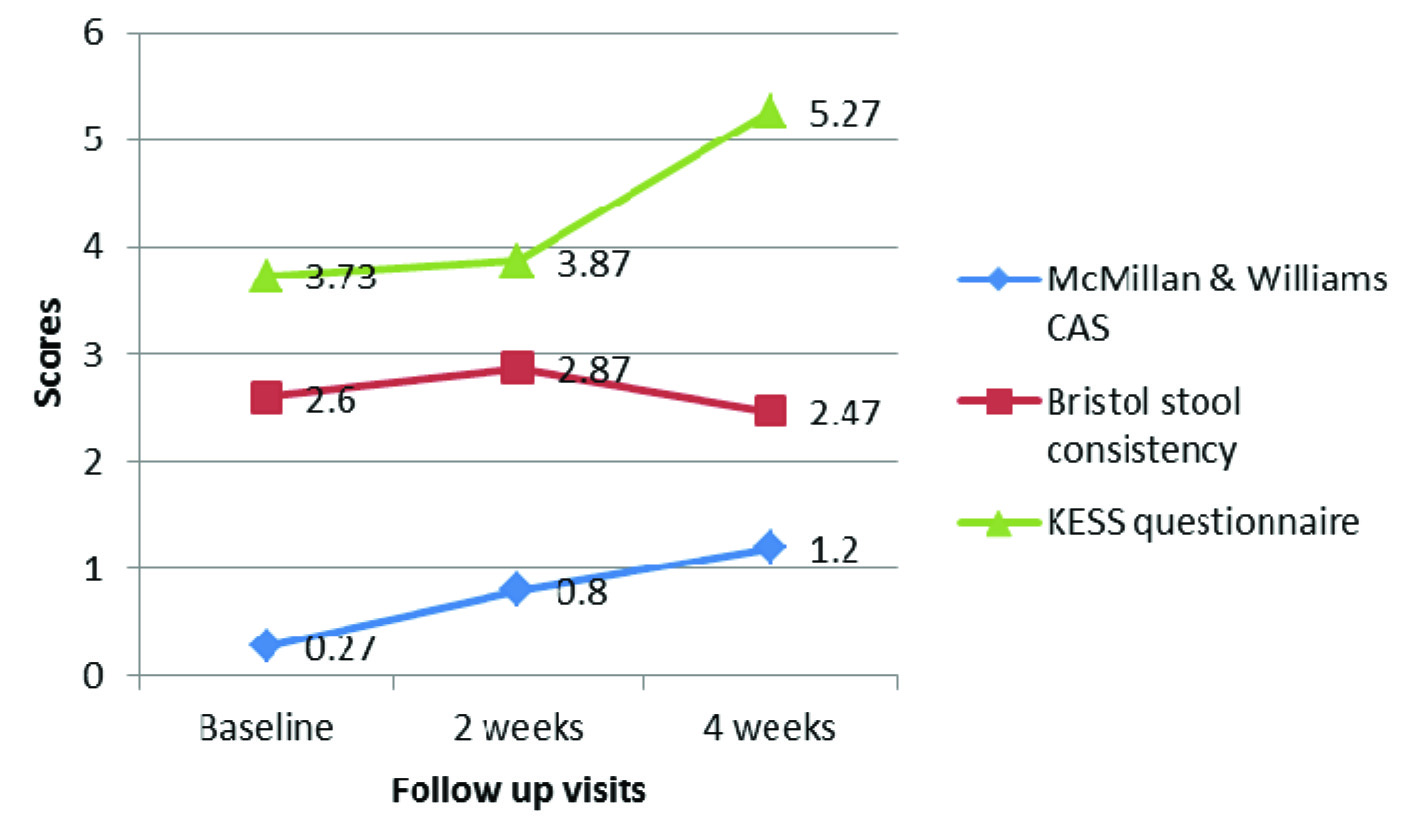
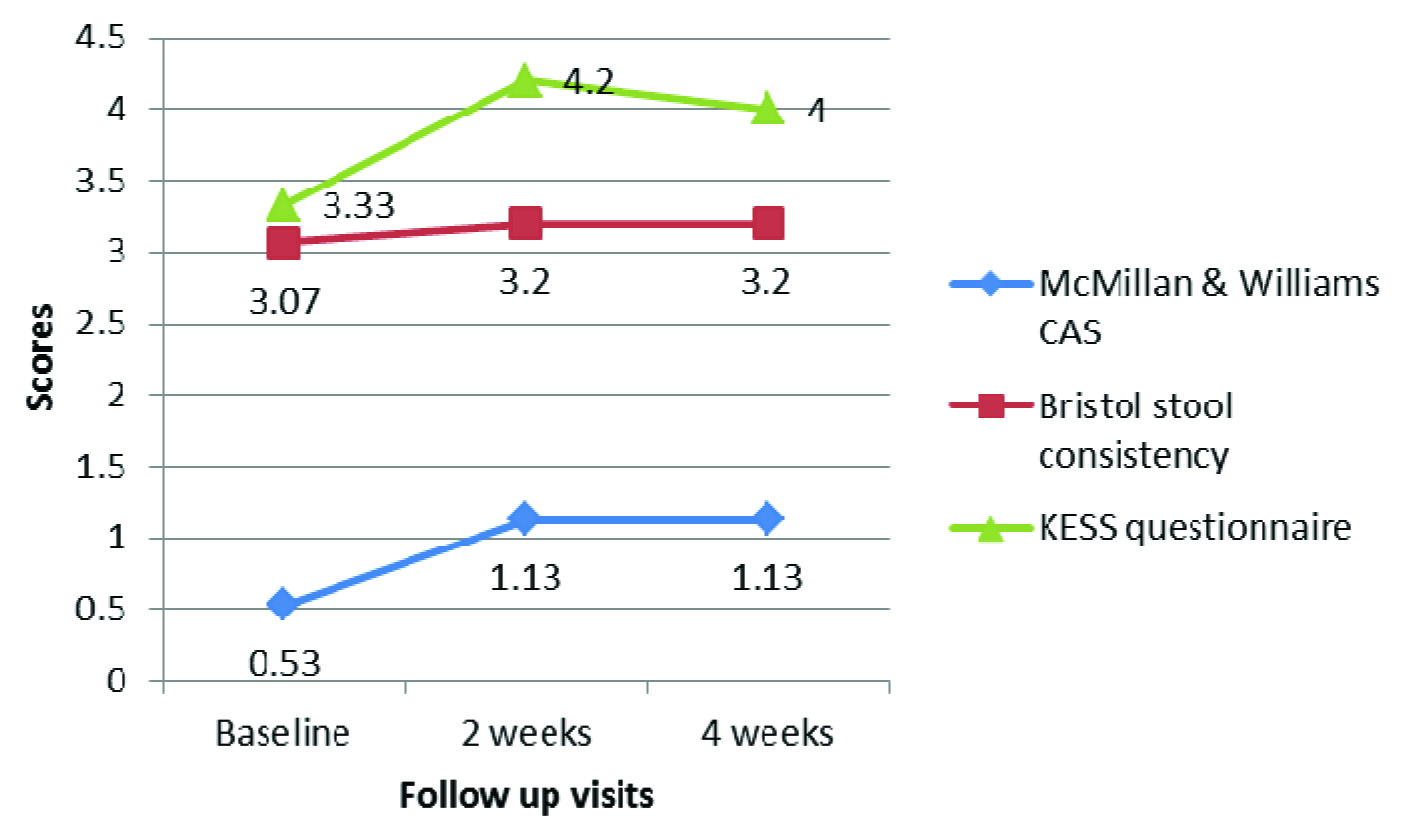
The stool consistency measured as per Bristol stool chart showed a decrease in consistency of stool in darifenacin group between baseline and two weeks, and two weeks and four weeks, which was statistically significant (p = 0.021 and 0.032 respectively).
However, the difference was not significant between baseline and 4 week assessment (p = 0.566). The consistency of stool in patients with trospium too decreased slightly between baseline and follow up observations, though not statistically significant (p = 0.076). There was no significant difference in scores of KESS questionnaire between baseline, 2 weeks and 4 weeks, both within the group and between the groups (p > 0.05). A statistically significant increase in McMillan & Williams CAS was observed in both the groups between baseline and 2 weeks, and baseline and 4 weeks (p < 0.05), indicating troublesome defecation with the use of study medications. However, there was no significant difference between week 2 and week 4 McMillan & Williams CAS (p > 0.05) [Table/Fig-4,5,6]. Also, the difference in CAS between the two groups was not statistically significant either at week 2 or week 4 of treatment (p = 0.778 and p = 0.944 respectively). All the patients showed good compliance to the study medications (as assessed using pill count method).
Comparison of efficacy and tolerability measures between and within the treatment groups.
| Darifenacin | p-value | Trospium | p-value (comparison within the group) | p-value (comparison between 2 groups) |
|---|
| Baseline | 2 weeks | 4 weeks | Baseline | 2 weeks | 4 weeks |
|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
|---|
| OABSS | 9.87 ± 3.75 | 4.07 ± 3.21 | 4.07 ± 3.29 | 0.0005* 1.000† 0.0005‡ | 9.27 ± 3.21 | 4.27 ± 3.70 | 4.00 ± 3.86 | 0.0005* 0.063† 0.0005‡ | 2 weeks | 0.952• |
| 4 weeks | 0.654• |
| Bristol stool consistency | 2.60 ± 0.97 | 2.87 ± 0.82 | 2.47 ± 0.97 | 0.021* 0.032† 0.566‡ | 3.07 ± 0.87 | 3.20 ± 1.24 | 3.20 ± 1.24 | 0.076§ | 2 weeks | 0.227• |
| 4 weeks | 0.017• |
| KESS questionnaire | 3.73 ± 3.45 | 3.87 ± 4.65 | 5.27 ± 5.98 | 0.084§ | 3.33 ± 4.41 | 4.20 ± 4.44 | 4.00 ± 4.56 | 0.053* 0.157† 0.105‡ | 2 weeks | 0.858• |
| 4 weeks | 0.244• |
| McMillan & Williams CAS | 0.27 ± 0.58 | 0.80 ± 1.63 | 1.20 ± 2.01 | 0.032* 0.063† 0.007‡ | 0.53 ± 1.17 | 1.13 ± 1.89 | 1.13 ± 1.89 | 0.011* 1.000† 0.011‡ | 2 weeks | 0.778• |
| 4 weeks | 0.944• |
*For comparison between baseline and 2 weeks (Wilcoxon signed ranks test)
†For comparison between 2weeks and 4 weeks (Wilcoxon signed ranks test)
‡For comparison between baseline and 4 weeks (Wilcoxon signed ranks test)
§For overall comparison between baseline, 2 weeks and 4 weeks (Friedman test)
•Mann Whitney U-test
Changes in constipation assessment scores at different visits (Darifenacin group)
Changes in constipation assessment scores at different visits (Trospium group)
Discussion
In the present comparative study, darifenacin 7.5 mg decreased the mean scores of day time frequency by -0.80 (80%), nocturia by -0.87 (31%), urgency by -1.87 (55%), UUI by -2.27 (85%) and OABSS by -5.80 (~59%) during the 4 week study period. A systematic review by Lam S et al., showed that darifenacin 7.5 mg reduced the daytime urinary frequency by -1.6 to -1.9 episodes / day, and UUI by -8.2 to -10.4 / week [3]. Another study evaluating the efficacy of darifenacin 7.5 mg in patients aged ≥65 years showed an overall decrease of -25.3% in frequency and -88.6% in UUI episodes during the 12 week study period [14]. Study published by Jha et al., showed that darifenacin decreased urinary frequency and urgency episodes / day by -1.6 (16.6%) and -2.0 (29%), and decreased the number of UUI episodes / week by -8.8 (68.4%) [7]. In the present study, trospium 60 mg decreased the scores of day time frequency by -0.47 (~87%), nocturia by -0.93 (~41%), urgency by -1.47 (62%), UUI by -1.47 (~57%) and OABSS by -5.27 (~57%). Study conducted by Zinner et al., [6], evaluating the efficacy of trospium 20 mg OD showed a decrease of -2.4 and -15.4 in the episodes of frequency / day and UUI / week respectively, during the 12 week treatment period. Systematic review by Lam S et al., observed that trospium 20 mg OD decreased the number of episodes of frequency / day and UUI / week by -2.7 and -16.1 respectively, during the 12 week treatment period [3].Nocturia decreased by -0.8 in one of the studies with trospium 60 mg during the 12 week treatment period [15]. A direct comparison of efficacy of the two drugs with the earlier reports could not be made as the scoring system used in the present study (OABSS) was different from that of the previous studies. However, statistically significant improvements in the component symptoms of OAB were observed with the two drugs in earlier studies and the same has been documented in the present study too.
Since the majority of constipation cases with darifenacin 7.5 mg are known to occur within 2 weeks of treatment initiation [8], a study duration of 4 weeks was chosen for the present study. The Bristol stool chart assessment in the present study showed a decrease in consistency of stool over the study period, indicating that there was no unfavorable alteration in stool consistency either with darifenacin or trospium. However, a transient alteration in food habits or increased water intake due to antimuscarinic induced dry mouth might have favorably altered the consistency of stool. Furthermore, the 4th week consistency of stool in patients with trospium was significantly lower than that of darifenacin (p = 0.017), which could be attributed to its higher baseline Bristol stool consistency score. KESS questionnaire assessments showed a nominal rise in scores between baseline and follow up visits in both the groups. However, there was no significant worsening or difficulty in defecation. The McMillan and Williams CAS showed a significant rise in scores between baseline and 2 weeks and baseline and 4 weeks in both the study groups. The discrepancy in the results of two scoring systems can be explained by the following reason. While KESS questionnaire measures the increasing frequency of various manifestations of constipation with the use of study medications using a detailed 11 item questionnaire, the McMillan and Williams CAS measures the impact of these symptoms on patient’s well-being, likely to be adversely affected due to altered gastrointestinal motility, as the response to each question / item in it is measured in terms of no problem, some problem or severe problem. Thus, though there was a marginal change in constipation symptom frequency and difficulty with darifenacin and trospium, their impact on patient well-being was significant. However, darifenacin and trospium did not significantly differ from each other in their propensity to cause difficult defecation.
The present comparative study of darifenacin and trospium shows that both the drugs are comparable in their efficacy. Similar observations have been made in previous studies [16]. A multicentre, double-blind study enrolling 561 patients showed that darifenacin 7.5mg showed 67.7% improvement in OAB symptoms at the end of 12 weeks [17]. The present study showed an improvement of 58.76%, indicating that the maximum improvement was seen in the first 4 weeks of treatment. The occurrence and severity of constipation, and their impact on patient well-being was assessed using three different scoring systems. The assessment of stool consistency showed no worsening or increase in stool consistency. None of the patients requested withdrawal from the study, prescription of any laxatives or stool softening medications, indicating good patient acceptability and tolerability with both the drugs. Results from a pooled phase III study has shown the incidence of constipation to be 14.8%, with 0.6% opting for treatment discontinuation and 3.3% requiring concomitant laxatives with darifenacin 7.5 mg [8]. Another study showed the occurrence of constipation to be at 18.6% in those ≥ 65yr and 13.3% in those between 18-64 years [8]. A study assessing the correlation between the drug therapy of OAB and incidence of constipation has shown that the odds of experiencing constipation with darifenacin 7.5mg was 1.93 and with trospium 60 mg was 2.93, when compared to placebo [18].
This is the first study to the best of our knowledge with a head to head comparison between darifenacin and trospium for their efficacy and tolerability in terms of constipation. As discussed above, most of the previous studies have presented the data on occurrence of constipation, whereas, the present study has made an attempt to assess, in detail, the constipation severity, stool consistency and its impact on patient comfort in passing stools and daily routine with the use antimuscarinics for OAB. Multicentric studies with larger sample size and longer study duration may be undertaken on similar lines to add more to the existing data on tolerability of antimuscarinics, especially with the population at risk of developing troublesome antimuscarinic side effects.
Conclusion
Both darifenacin and trospium effectively alleviate the symptoms of overactive bladder and are comparable in tolerability with respect to constipation, with a moderately good patient acceptability.
*For comparison between baseline and 2 weeks (Wilcoxon signed ranks test)
†For comparison between 2weeks and 4 weeks (Wilcoxon signed ranks test)
‡For comparison between baseline and 4 weeks (Wilcoxon signed ranks test)
§For overall comparison between baseline, 2 weeks and 4 weeks (Friedman test)
•Mann Whitney U-test