Kodameae ohmeri is an emerging pathogen in various types of infections. Most infections are seen in patients with compromised immunity like cancer patients. Few cases of neonatal infections due to K. ohmeri have been reported earlier in premature neonates with fatal outcomes. We report two cases of fungemia; the first case was a patient with hematological malignancy, who complained of fever spikes and grew K. ohmeri in blood despite prophylactic voriconazole therapy. The second case was in a mature neonate, who developed respiratory distress and features of sepsis two days after birth, multiple blood cultures were positive for K. ohmeri. Both the patients responded well to Amphotericin B. Repeat blood cultures were sterile and patients were discharged.
K. ohmeri is an unusual and emerging fungal pathogen of late an increasing number of cases of fungemia, funguria, endocarditis, peritonitis and wound infections due to the same are being reported. Some occur in immunocompromised patients and some inapparently immunocompetent patients, neonates with an inclination for preterm babies. We report two case of fungemia, one with lymphoma and the second in a neonate.
Case Report
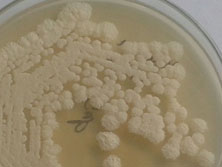
A 50-year-old male patient was diagnosed with Large B cell lymphoblastic lymphoma. After receiving three cycles of chemotherapy with high dose cytoarabinoside + methotrexate+ endoxan presented to the medical oncology department of our hospital for further management. He was a known case of type 2 diabetes mellitus. On examination, he was conscious, oriented, afebrile, with pallor, generalized weakness, bilateral lower limb paraesthesia, hepatosplenomegaly and supraclavicular and inguinal lymphadenopathy. MRI and PET CT suggested intracranial metastasis and the patient was given first cycle of second line chemotherapy (R-ICE: Ritximab, Iphosphamide, carboplatin and Etoposide). After the first cycle he developed pancytopenia, Hemoglobin was 7.6g/dl, WBC count was 0.04 x 103/μL and platelet count was 9 x 103/μL. He was transfused with packed cells and platelets and empiric voriconazole therapy was started as antifungal prophylaxis. Before initiating second cycle chemotherapy, he had fever spikes. Blood cultures were sent in BacT/Alert FA Plus bottle, (BacT/Alert 3D - BioMerieux® Marcy l’ Etiole -France) which grew budding yeast cells. This was subcultured on Saboraud’s dextrose agar and Chrome agar Candida (HiMedia, Mumbai, India). On Saboraud’s dextrose agar white fluffy colonies grew [Table/Fig-1] and on Chrome agar Candida the color of the colonies changed from pink to blue [Table/Fig-2] within 48h. The yeast was identified by Vitek-2 compact (BioMerieux® Marcy l’ Etiole -France) using VITEK2 YST card as Kodameae ohmeri, with 99% identification. The isolate was sent for molecular confirmation to PGIMER, Chandigarh. The ribosomal DNA (rDNA) of the two isolates was amplified by polymerase chain reaction (Bangalore Genie) and sequenced using the BigDye terminator cycle (Applied Biosystems, Foster City, CA). Sequence analysis of the 28SrRNA region was done on Genetic Analyzer 3130 (Applied Biosystems)].
White fluffy colonies on Saboraud’s dextrose agar
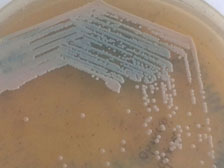
Colonies changing from pink to blue over 48hours on Chrome agar Candida
Antifungal susceptibility was done using VITEK2 AST-YS07 card. The isolate was sensitive to Amphotericin B (MIC=0.25), Flucytosine (MIC=1), Caspofungin (MIC=0.25), Voriconazole (MIC=0.25) and Fluconazole (MIC=1). Despite on Voriconazole prophylaxis and in-vitro sensitivity to voriconazole the patients developed fungemia due to K. ohmeri. After initiating Amphotericin B therapy the patient had uneventful recovery. Repeat blood culture was sterile. After the completion of chemotherapy cycles the patient was discharged.
Case 2
A term female baby was born by normal vaginal delivery and cried immediately after birth. No active resuscitation was required. Her Apgar score was 8. She developed respiratory distress day two onwards which was progressive in nature. She was treated with Injection ceftriaxone and sodium bicarbonate and shifted to the neonatal intensive care unit for further management. On general examination, baby’s heart rate was 160/min; respiratory rate 90/min, oxygen saturation was 91% on room air. Pulses were feeble. She had nonblanching pink petechiae and acrocyanosis. Anterior fontanelle measured 1.5x1.5 cm.
Due to respiratory distress baby was intubated same day, fluid bolus was given and mechanical ventilation started. Ionotropic support (Dobutamine) started along with Fentanyl, midazolam drip and other supportive treatments. Intravenous antibiotics were given namely piperacillin-tazobactam and amikacin. Baby was extubated after 48h, that is, on day 3. However, immediately after extubation distress worsened, baby developed features of sepsis, for which she was re-intubated and ventilated again. Her WBC count was 11.73x 103/μL, with differential count showing 71% polymorph nuclear cells, 20% lymphocytes, 1.9% eosinophils, 6.1% monocytes and 0.3% basophils. Platelet count was 169x103/μL. C - reactive protein was 6mg/L and absolute neutrophil count was 8.3x103/μL. Blood culture was sent in BacT/Alert FP Plus bottle after reintubation, which flagged positive within 12 h; smear showed budding yeast cells, was immediately informed to the neonatologist and the baby was started on IV Fluconazole. The yeast cell was identified as K. ohmeri by Chrome agar Candida (HiMedia, Mumbai, India) and VITEK2YST card (BioMerieux® Marcy l’ Etiole -France). A second blood culture was sent on day 4, which also grew K. ohmeri. The isolate was intermediately sensitive to Fluconazole (MIC=4) and sensitive to Amphotericin B (MIC=0.25), Flucytosine(MIC=1), Caspofungin (MIC=0.25) and Voriconazole (MIC=0.25). Intravenous Amphotericin B was started on day 5 and continued for 21 days and the baby responded well. A repeat blood culture showed no growth. Baby was discharged. Mother’s high vaginal swab also grew budding yeast cells, which was later identified as the same, confirming that probably baby got the infection during parturition.
Discussion
The epidemiology of fungal pathogens has changed over a period, with new species emerging as well as old species increasingly becoming more virulent and resistant to antifungal treatment. So has changed the clinical spectrum. One such emerging pathogen is K. ohmeri [1]. Of late a number of cases have been reported due to the same causing fatality unless identified and treated appropriately from India [2,3].
K. ohmeri, the fungus in current discussion is emerging ascomycetous yeast. The taxonomy has changed, earlier it was known as Pichia ohmeri/ Yamadazyma ohmeri. It is the telemorph of Candida guillermondii var membranaefaciens [4].
Infections due to K. ohmeri occur in patients of different age groups and immune profiles. The patient group includes neonates and children, immunocompromised cancer patients, patients with other chronic illness such as diabetes as well as immunocompetent patients [1,3,5–10]. A case of fungemia caused by K. ohmeri in a 3-year-old female patient was reported by de Barros et al., from Brazil [9]. It was also reported to cause outbreak in paediatric intensive care unit [11]. Although majority of cases reported are fungemia (as in the indexed patients), infections of other sites have also been documented [2,12–18]. We reported a case of funguria due to K. ohmeri in a diabetic patient earlier [13]. We have also isolated the same from three urine samples (probably colonizers), one from a catheter tip (Unpublished data). More recently Menon T et al., described one case of oral candidiasis due to K. ohmeri in a 38 y HIV positive female patient, which was identified by the API 20 C yeast identification system and confirmed by sequence analysis [19]. Son JS et al., reported a case of malignant external otitis [20]. These case reports implicate the varied clinical presentation of this emerging yeast.
In one of the studies, review of previous cases suggests, central venous catheters being a major predisposing factor [12,21]. K. ohmeri fungemia associated with colonoscopic stent insertion has recently been reported by Yu TS et al., [22]. Postsurgery fungemia has also been reported [17,23]. Broad spectrum antibiotics use, especially piperacillin-tazobactam use has been significantly associated with K. ohmeri fungemia [12]. Parenteral therapy is a noted risk factors for infections due to K. ohmeri [24]. Kodameae has been recovered from fungemia cases in patients with hematological malignancy like our first case [1,17,10,11]. Neutropenia possibly predisposed the patient for fungemia [1,17,11]. In neonates prematurity is possibly a predisposition, though our case was a term baby [3,7,8]. She was delivered through normal vaginal route, and developed features of respiratory distress, sepsis, multiple blood cultures were positive, so we consider this as a significant pathogen. Moreover the mother also grew the same yeast from high vaginal swab, indicating the possibility that of the infection was possibly acquired during parturition. Signs and symptoms are nonspecific depends upon the site of infection however fever is a usual association.
Identification of Kodameae in a routine laboratory using conventional methods is not always possible. On Saboroud’s dextrose agar it grows as white fluffy colonies; it may variably be reported as Candida tropicalis or Candida hemolounii by conventional methods [12,24]. Chrome agar Candida can be of value in provisional identification as the colour of K. ohmeri colonies changes from pink to blue in a span of 48h [25]. Automated identification systems like API 20C, Vitek 2 ID YST and Microscan along with molecular methods are the mainstay of laboratory identification of this rare fungus [1,24]. Molecular identification is done by PCR amplification followed by sequencing of 18S rRNA, the D1/D2 domains of 26SrRNA, the internal transcriber spacer 1/2 (ITS) of the ribosomal DNA,28SrRNA and/or 5.8SrRNA [5,7,9, 12,18, 24]. Lee et al., used pulsed field gel electrophoresis for karyotyping. Restriction endonuclease analysis of NotI-digested DNA (REAG-N) is useful for genotyping the clinical isolates of K. ohmeri [25]. Fluorescent amplified fragment length polymorphism was used for molecular typing by Chakrabarti et al., [12].
Susceptibility pattern of K. ohmeri is difficult to comment on as there is a paucity of cases reported so far. However, in vitro resistance to Fluconazole has been reported [18]. According to the review by Shang et al., majority of patients receiving fluconazole have succumbed [1]. These evidence suggest that azoles are probably ineffective against K. ohmeri and should not be used as first line antifungal agent. Though Shang et al., suggested voriconazole and echinocandins as optimal therapy, in our first case the patient was on Voriconazole prophylaxis, yet developed fungemia, though in-vitro it was susceptible [1]. In the second case there MIC of fluconazole was raised and it showed intermediate susceptibility. On the other hand, both of them responded well to amphotericin B In our view the most appropriate antifungal would be amphotericin B, which is also in accordance with other studies [1,26]. Echinocandins are recently introduced novel antifungal agents and very few studies investigating their clinical efficacy in patients with K. ohmeri infections are available. K. ohmeri fungemia has been successfully treated with caspofungin and micafungin in two cases, suggesting echinocandins as good alternatives [6,26,27].
Conclusion
With the advances in healthcare management and the increasing range of uncommon yeast pathogens, identification of Candida sp as albicans or non-albicans no more suffices. Correct identification upto species level, interpreting the significance as a pathogen along with antifungal susceptibility results is necessary for best clinical outcome.
[1]. Shang ST, Lin JC, Ho SJ, Yang YS, Chang FY, Wang NC, The emerging life-threatening opportunistic fungal pathogen Kodamaea ohmeri: optimal treatment and literature reviewJ Microbiol Immunol Infect 2010 43:200-06. [Google Scholar]
[2]. Sundaram PS, Bijulal S, Tharakan JA, Antony M, Kodamaea ohmeri tricuspid valve endocarditis with right ventricular inflow obstruction in a neonate with structurally normal heartAnn Pediatr Cardiol 2011 4:77-80. [Google Scholar]
[3]. Poojary A, Sapre G, Kodamaea ohmeri Infection in a NeonateIndian Pediatrics 2009 46:629-31. [Google Scholar]
[4]. Yamada Y, Suzuki T, Matsuda M, Mikata K, The phylogeny of Yamadazyma ohmeri (ETCHELLS et BELL) BILLON-GRAND based on the partial sequences of 18s and 26s ribosomal RNAs: The proposal of Kodameae Gen. Nov. (Saccharomycetaceae)Biosci Biotech Biochem 1995 59(6):1172-74. [Google Scholar]
[5]. Santino I, Bono S, Borruso L, Bove M, Cialdi E, Martinelli D, Kodamaea ohmeri isolate from two immunocompromised patients: first report in ItalyMycoses 2013 56:179-81. [Google Scholar]
[6]. Shaaban H, Choo HF, Boghossian J, Perez G, Kodamaea ohmeri fungemia in an immunocompetent patient treated with micafungin: case report and review of the literatureMycopathologia 2010 170(4):223-28. [Google Scholar]
[7]. Taj-Aldeen SJ, Doiphode SH, Han XY, Kodamaea (Pichia) ohmeri fungaemia in a premature neonateJ Med Microbiol 2006 55:237-39. [Google Scholar]
[8]. Borade Ashwin, Kapdi Muznah, Suryavanshi Kalpana, Kodamaea Ohmeri –An Emerging Fungal Pathogen in Neonatal Intensive Care Unit. DOI: 10.7199/ped.oncall 2014 66 [Google Scholar]
[9]. De Barros JD, Do Nascimento SM, De Araujo FJ, Braz RF, Andrade VS, Theelen B, Kodamaea (Pichia) ohmeri fungemia in a pediatric patient admitted in a public hospitalMed Mycol 2009 47:775-79. [Google Scholar]
[10]. Mahfouz RA, Otrock ZK, Mehawej H, Farhat F, Kodamaea (Pichia) ohmeri fungaemia complicating acute myeloid leukaemia in a patient with haemochromatosisPathology 2008 40:99-101. [Google Scholar]
[11]. Otag F, Kuyucu N, Erturan Z, Sen S, Emekdas G, Sugita T, An outbreak of Pichia ohmeri infection in the paediatric intensive care unit: case reports and review of the literatureMycoses 2005 48:265-69. [Google Scholar]
[12]. Chakrabarti A, Rudramurthy SM, Kale P, Hariprasath P, Dhaliwal M, Singhi S, Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centreClinl Microbiol Infect 2013 DOI: 10.1111/1469-0691.12337 [Google Scholar]
[13]. Sahu M, Bhalekar P, Keny D, Fungiuria due to K. ohmeri in a diabetic: A case ReportJournal of Medical Research and Practice 2013 2(6):134-35. [Google Scholar]
[14]. Puerto JL, García-Martos P, Saldarreaga A, First report of urinary tract infection due to Pichia ohmeriEur J Clin Microbiol Infect Dis 2002 21:630-31. [Google Scholar]
[15]. Reina JP, Larone DH, Sabetta JR, Krieger KK, Hartman BJ, Pichia ohmeri prosthetic valve endocarditis and review of the literatureScand J Infect Dis 2002 34:140-41. [Google Scholar]
[16]. Choy BY, Wong SS, Chan TM, Lai KN, Pichia ohmeri peritonitis in a patient on CAPD:response to treatment with amphotericinPerit Dial Int 2000 20:91 [Google Scholar]
[17]. Xiao Y, Kang M, Tang Y, Zong Z, Zhang Y, He C, Kodamaea ohmeri as an Emerging Pathogen in Mainland China: 3 Case Reports and Literature ReviewLab Med 2013 44:e1-e9. [Google Scholar]
[18]. Yang BH, Peng MY, Hou SJ, Sun JR, Lee SY, Lu JJ, Fluconazole-resistant Kodamaea ohmeri fungemia associated with cellulitis: case report and review of the literatureInt J Infect Dis 2009 13:e493-97. [Google Scholar]
[19]. Menon T, Herrera M, Periasamy S, Palanivelu V, Sikhamani R, Wickes B, Oral candidiasis caused by Kodamaea ohmeri in a HIV patient in Chennai, IndiaMycoses 2010 53:458-59. [Google Scholar]
[20]. Son JS, Shin SY, Min TH, A case of malignant external otitis with Pichia ohmeriKorean J Infect Dis 2002 34:349-53. [Google Scholar]
[21]. Hitomi S, Kumao T, Onizawa K, Miyajima Y, Wakatsuki T, A case of central-venous-catheter-associated infection caused by Pichia ohmeriJ Hosp Infect 2002 51:75-77. [Google Scholar]
[22]. Yu TS, Lee JY, Park YM, Choi HK, Kim YK, Kim HY, Kodamaea ohmeri Fungemia associated with Colonoscopic Stent Insertion: A Case ReportKorean J Med 2013 85(1):106-09. [Google Scholar]
[23]. Garcia-Tapia A, Garcia-Agudo R, Marin P, Conejo JL, Garcia-Martos P, Kodamaea ohmeri fungemia associated with surgery [Article in Spanish]Rev Iberoam Micol 2007 24:155-56. [Google Scholar]
[24]. Lee JS, Shin JH, Kim MN, Jung SI, Park KH, Cho D, Kodamaea ohmeri isolates from patients in a university hospital: identification, antifungal susceptibility, and pulsed-field gel electrophoresis analysisJ Clin Microbiol 2007 45:1005-10. [Google Scholar]
[25]. Agrawal V, Bhagwat AM, Vishalakshi V, Gode V, Sawant CS, Exploring the potential of chromogenic medium for the identification of medically important yeast species other than CandidaInt J Pharm Pharm SciVol 6(Issue 3):291-94. [Google Scholar]
[26]. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. ESCMID/ECMM Joint Clinical Guideline for the Diagnosis and Management of Rare Invasive Yeast Infections Clinical microbiology and infection. doi: 10.1111/1469-0691.12360 [Google Scholar]
[27]. Chiu CH, Wang YC, Shang ST, Chang FY, Kodamaea ohmeri fungaemia successfully treated with caspofunginInt J Antimicrob Agents 2010 35:98-99. [Google Scholar]