The term “accessory pancreatic duct (APD)” doesn’t mean extra, but an important “additional” duct lying within the head of the pancreas. It drains most of the upper third and superficial part of the lower two thirds of the head and uncinate process of the pancreas [1]. Developmentally, APD represents the persistent proximal part of the dorsal pancreatic duct, transporting pancreatic secretion from the larger dorsal pancreatic primordia [2]. This small calibered duct has inverse relation with its secretory capacity. In addition, the duct may not be patent in all causing the risk of pancreatic pathology in susceptible individuals [3]. Many studies have reported variation in the accessory duct patterns [2,4–6]. Knowledge of such variations in their pattern and termination are important in cannulation of the minor duodenal papilla especially in pancreatic divisum and in distortion of major pancreatic duct (MPD), for the endoscopic diagnosis and treatment of pancreatic diseases [4]. Present study is aimed to evaluate the possible variations in the APD and its terminations.
Materials and Methods
This study was conducted on 40 formalin fixed adult human (33 male and 7 female) cadavers with age range between 35 to 50 years (as per our department record) in the Department of Anatomy, Kasturba Medical College, Manipal University, Manipal (India), during the period of March 2012 to June 2014. The dissection and removal of the duodenum and pancreas was carried out as per the standard protocol [7]. On the posterior surface of the isolated pancreas, two parallel cuts were made close to the superior and inferior margins of the body. The lobules of the gland were picked between the cuts to expose the greyish white pancreatic duct system. The accessory pancreatic duct was first located in the body of the gland and dissected by piece meal dissection towards its duodenal opening and also traced towards its connection with main pancreatic duct. Later, 1% aqueous solution of eosin was injected through both the duct. Both the duodenal papillae were identified and appearance of the colored fluid indicating patency of the pancreatic duct was also recorded. Subsequently the accessory duct was dissected in the body of the gland towards the minor papillae, following the tributaries for a short distance into the substance of the gland.
Results
The accessory pancreatic duct was found to be present in 38 out of 40 specimens and the remaining two specimens showed embryonic type of duct system. The long type accessory duct [Table/Fig-1] was seen in 20 (50%) specimens, which began at the junction of head with the body of pancreas and passed transversely to the right anterior to the CBD towards the minor duodenal papilla. Out of 20 cases, 15 (75%) specimens showed patent orifice at minor papilla and in 25% of specimens the duct was obliterated as fibrous structure. The short type ducts [Table/Fig-2] were observed in 9 (22.5%) specimens, which began in the head and ascended anterior to the CBD to terminate in the minor duodenal papilla. In short type, five ducts were obliterated and two ducts were patent at its minor papilla. In two (5%) specimens the duct terminated within the substance of the gland. Similarly, the ansa pancreatica type [Table/Fig-3] in 9 (22.5%) specimens began in the head and descended to the lower part of head forming a loop with convexity towards inferior aspect. It then ascended anterior to major duodenal papillae and CBD to open onto the minor papilla. In this type, four ducts had patent orifice and five ducts were obliterated towards its duodenal end. Overall, patency of the APD was noted in 21 (52.5%) and absent in 17 (42.5%) specimens. Thirty six (90%) of the accessory ducts were terminated on to the minor duodenal papillae, two (5%) ducts disappeared within the substance of pancreas and the rest two (5%) specimens showed embryonic type of duct pattern, in which the dorsal and ventral pancreatic ducts did not fuse and open separately onto the minor and major duodenal papilla respectively.
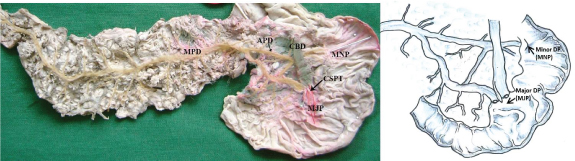
(Posterior view) Photograph and schematic diagrams to show the long-type accessory pancreatic duct (APD) which joined the main pancreatic duct (MPD) in the neck portion and ran straight from the upper dorsal pancreatic duct. [CBD, common bile duct; MNP/minor DP, minor duodenal papilla; MJP/major DP, major duodenal papilla; CSPT, complete septum]
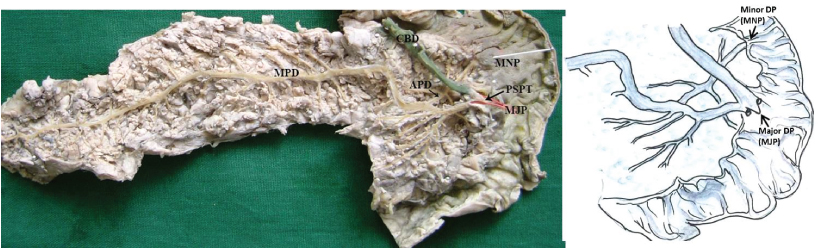
(Posterior view) Photograph and schematic diagrams to show the short-type accessory pancreatic duct (APD) which joined the main pancreatic duct (MPD) near the inferior branch and ran a descending course. [CBD, common bile duct; MNP/minor DP, minor duodenal papilla; MJP/major DP, major duodenal papilla; PSPT, partial septum]
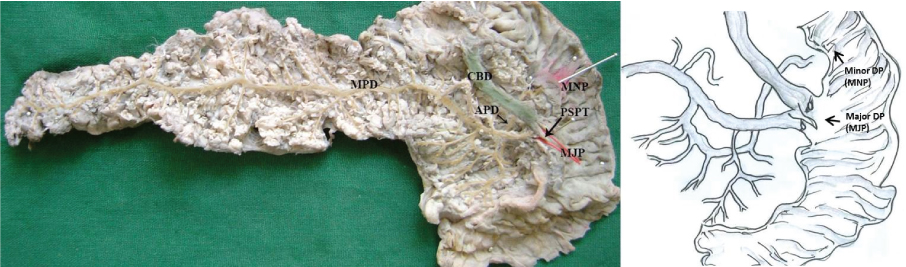
(Posterior view) Photograph and schematic diagrams to show the ansa pancreatica type of accessory pancreatic duct (APD). [CBD, common bile duct; MNP/minor DP, minor duodenal papilla; MJP/major DP, major duodenal papilla; PSPT, partial septum]
CBD, common bile duct; MNP/minor DP, minor duodenal papilla; MJP/major DP, major duodenal papilla; PSPT, partial septum separation; CSPT, complete septum separation
Careful observation of the terminal shape of the accessory pancreatic duct towards its opening into minor papilla shows gradually narrowing stick type termination in 56.52%, spindle shaped termination in 20%, saccular termination in 12.5%, and branching type of termination seen in 7.5%.
Discussion
Developmentally, both dorsal and ventral pancreatic buds were drained independently by two separate ducts. With further growth and rotation, the distal part of the dorsal pancreatic duct merge with the ventral pancreatic duct to form main pancreatic duct (MPD, Wirsung’s duct) and the proximal part of the dorsal pancreatic duct partially regresses to form the accessory pancreatic duct (APD, Santorini’s duct) [8].
Dorsal pancreatic bud, being a larger part of the developing pancreas its duct drains most of the secretion. The dorsal pancreatic duct is smaller than its counterpart and drains most of the secretion through the minor duodenal papillae, which is smaller than the major duodenal papillae. Secondly, inverse relationship of accessory pancreatic duct with its secretory capacity might presumably put significant load on the minor duodenal papillae. For this reasons, a patent APD is essential to reduce the pressure in the MPD by acting as a secondary drainage and prevent the development of acute pancreatitis [4]. Literature survey suggested the gross variation in the incidence of patent APD from 12% [9] to 82% [10], which could be due to difference in the number of specimens used or due to the mode of survey conducted. In contrast, our study revealed that the accessory duct was patent in 52.5% of specimens, which is similar to the study of Gosavi and Gaikwad [11] by dissection method (in 60%) and in ERCP studies by Dawson and Langman showed patency in 42.5% [6].
Long type of APD followed a straight course, representing the continuation of the main dorsal pancreatic duct. It joins the MPD at the neck portion of the pancreas, carrying more pancreatic secretion through a relatively smaller sphincter at minor papilla [2]. In such case even a minor papillary insufficiency or narrowing would obstruct the dorsal pancreatic duct, causing retention of secretion, and results in pancreatitis [5]. Our study revealed 50% of the accessory ducts were of long type. Of which 75% of specimens presented patent APD, which means long type accessory ducts are more likely to have patent orifice at its duodenal end. Dissection study by Gosavi and Gaikwad [11] found 74% long type ducts and ERCP study by Kamisawa and Okamoto [1] showed 66.8% of similar duct pattern.
We found 22.5% of accessory ducts belonged to the short type, which represents the fusion of proximal part of the main dorsal pancreatic duct and its inferior branch with the main pancreatic duct. Short type ducts presents 22.5% of patent ducts, 55% of obliterated ducts towards its minor papilla, and remaining 22.5% of ducts terminated in the gland itself. This finding proves that the short type ducts are less likely to have patent orifice. Kamisawa and Okamoto found 33.5% of short ducts. Compared with earlier studies, Dawson and Langman found obliteration of the short type accessory ducts in 30% of cases, and Gosavi and Gaikwad found in about 38% of their cases.
The present study revealed 22.5% of the accessory ducts were found to be ansa pancreatica type, which is formed by the most proximal part of the dorsal pancreatic duct and its inferior tributary united with the inferior tributary of the ventral pancreatic duct [2]. Earlier studies by Gosavi and Gaikwad had 24% and Dawson and Langman found 17.5% [6,11]. Though it can be proposed that this ansa type of duct prevent the occupancy of pancreatitis by decreasing the pressure of main pancreatic duct, controversies still exists. In contrast to the other tributaries of the main pancreatic duct which join the main pancreatic duct at right angles, the ansa joins the main duct at an oblique angle. Because of this ductal anatomy, it has been proposed that the areas served by the ansa have poor drainage of pancreatic juice. In conditions such as alcoholism or functional stenosis of the sphincter of Oddi, the drainage of pancreatic juice is further impaired and such patients are vulnerable to the development of pancreatitis [12]. Our dissection study shows nearly half of the ansa types of accessory ducts were patent towards its duodenal end, but shows variable incidence in ERCP methods i.e., 33.3% in Dawson’s study [6] and 15% in Kamisawa study [2].
The embryonic type of accessory ducts where the dorsal and ventral pancreatic ducts failed to fuse, found in 5% of the cases studied. However, the incidence may vary from 2 to 7.5% [6,11,13,14]. It is characterised not only by the anatomical deformity but also by the physiology of duct drainage as its drainage is predominantly through the dorsal duct of Santorini into minor duodenal papillae. This disproportion between the small calibre of the minor papilla and the large amount of secretions from the dorsal part of the gland leads to a relative outflow obstruction from the dorsal pancreas leading to pain or pancreatitis. Thus pancreas divisum is thought to give rise to a spectrum of disease ranging from minor symptoms or chronic abdominal pain to acute relapsing or chronic pancreatitis [5].
The minor papilla was situated anterosuperior to the major papilla in 85% of the cases and the distance between the two papillae varied from 1.3 to 4.3 cm, with an average of 2.35 cm which is similar to the findings of the earlier studies [15]. This is particularly useful in case of difficult cannulation of papilla or absence of major papilla.
Kamisawa and his colleagues classified the accessory duct patterns into six types based on the terminal shape of the duct towards into minor papilla [4]. We found gradually narrowing stick type termination in 56.52%, spindle shaped termination in 20%, saccular termination in 12.5%, and branching type of termination seen in 7.5%. The terminal shape of the accessory duct predicts the patency of the duct. Our observations revealed that the stick type of duct termination was more patent than the rest of the types and branching type fail to show the patency test with aqueous eosin solution. The terminal shape of the APD could be correlated with the patency of the APD, which in turn function as a secondary drainage channel for the MPD and prevent the occurance of acute pancreatistis. Earlier literature suggests the patency of the spindle-type and cudgel-type APDs were more compared to the patency of the stick-type and saccular types resulting in fewer chances of pancreatic duct obstruction and pancreatitis. The branch-type of APD shows least patent or nil in most of the cases causing ductal obstruction and more chances of pancreatistis. An accessory duct depending on the degree of their luminal patency appears to trigger the event of acute pancreatitis, but not to the progression of pancreatitis [4].
Conclusion
Location of minor papilla and its distance from major papilla are important in difficult cannulation of the major papilla. Majority of the accessory ducts found in the study belonged to long type and were patent. Knowledge of the variations in the pancreatic duct system is essential for safe and effective diagnostic and therapeutic interventions.