Although bone support is very important in both function and aesthetics of dental implants, the soft tissues around an implant also play a direct role in aesthetics and function [1,2]. Therefore, indices evaluating tissue condition around implants and the amount of crestal bone loss have been assessed in many investigations [3–5]. Many factors may affect the peri-implant bone level the more important of which are bone quality, functional occlusal forces, parafunction, implant characteristics (size, shape and design), microbial factors and surgical technique [6].The latter is one of the most important factors in implant success; basically there are two approaches for surgery of implants namely one stage non submerged surgery or two stage or submerged surgery. Both techniques are useful in standard conditions; but the two stage technique is necessary in some cases (i.e. need for GBR) [7].
The effects of one stage surgery on implant bone loss or soft tissue characteristics have been evaluated in many studies. But some of them are animal studies [8] or their design is retrospective [9] and in other studies the one stage and two stage inserted implants are not similar in body design or crest module or thread distances [10]; which can influence results,especially the marginal bone loss.
The aim of this prospective study is to evaluate the soft tissue parameters and marginal bone loss around one stage and two stage implants. Our main hypothesis was that the non submerged implants may be as successful as submerged ones. This study was designed to evaluate changes in soft and hard tissues around one stage and two stage implants.
Materials and Methods
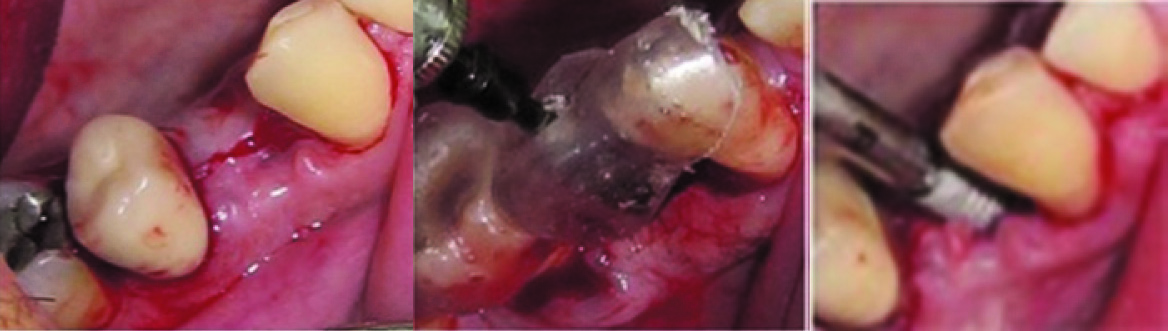
For this prospective cohort study 48 patients (22 males and 26 females aged 38 to 54 y with a mean age of 41.4±10.2 y) were selected after a screening examination that included a full medical and dental history,intraoral examination, full-mouth periodontal probing, and radiographs. The following inclusion criteria were used:1) plaque index<20%,2) presence of adjacent teeth, 3) stable occlusion, 4) presence of opposing teeth and 5) absence of caries in adjacent teeth. Patients had been received single implants from March 2012 to July 2012 and 4months after surgery, when the implants were loaded, 48 patients with implants in posterior mandible area were selected as follows: 24 patients with implants inserted in one stage protocol and 24 patients with two stage implants. Informed consent was signed by each of the participants after complete explanation of the surgery. The Committee of Ethics of Hamadan University of Medical Science approved the consent form and study protocol. All patients were non-smokers, periodontally and systemically healthy, were not taking medications known to interfere with periodontal tissue health or healing, with no contraindications for dental implants, and advised for proper oral hygiene (brushing for at least 5 min and using dental flosses and/or proxy-brushes). All surgical procedures were performed by one clinician and under standard conditions [Table/Fig-1]. The implants were TBR®, Connect (Implants Group, Toulouse, France) with conical bone level form and micro threads at the collar. All of the implants had at least 1mm of bone on the buccal and lingual sides and GBR treatment was not performed for any of them.During the one-stage surgery after inserting the implant healing abutments was installed and the flaps were adjusted to the implant and sutured while during the two-stage procedure the cover screw was placed on the implant and the flaps were sutured in a way that the implant was fully submerged. Three months later the implants were exposed and the cover screw was replaced with a healing abutment.There were no complications during surgery or healing period in patients and all the inserted implants had acceptable primary stability. The postsurgical periapical radiographs showed the implants shoulders were at the crestal bone level.
Implant inserting procedure
The following criteria were measured and recorded by one calibrated operator:
Sulcus probing depth (PD): Depth of sulcus was measured using a William’s probe in the mesial, distal and middle of buccal/lingual areas three times and its average was recorded as the probing depth around the implant.
Mucosal Thickness (MT): For measuring the thickness of gingiva an endodontic file number 20 was used. The file was inserted 1 mm apically under the sulcus and the distance between the tip of file and rubber stop was recorded as the mucosal thickness using a digital caliper [Table/Fig-2,3].
Width of keratinized gingival (KG): The KG width was measured using a William’s probe mid-facially from the gingival margin to the mucogingival junction of the implant.
Papilla Index(PI): The presence or absence of papilla was recorded using the Jemt index [11] as follows:
0: No papilla
1: Presence of papilla in less than 50 % of embrasures
2: Presence of papilla in more than 50 % but less than 100 % of embrasures
3: Presence of papilla in 100 % of embrasures
4: Presence of papilla in more than 100 % of embrasures
The index was recorded in mesial and distal areas of the implant and its average was assigned to that implant.
The distance between implant shoulder and alveolar crest (SC)The level of bone around the implant was calculated in stent guided parallel PA radiographs. The distance between implant shoulder and observed alveolar crest (at the bone to implant contact point) was recorded at the mesial and distal areas of implants by a digital caliper (with the precision of 0.01 mm) in millimeter and its average was recorded as the bone level for that implant [Table/Fig-4].
Evaluation of mucosal thickness using an endodontic file
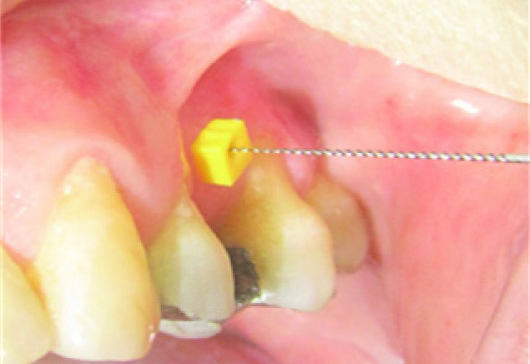
Measuring the distance between rubber stop and tip of the file using a digita caliper

Measuring the distance between the bone crest and implant shoulder
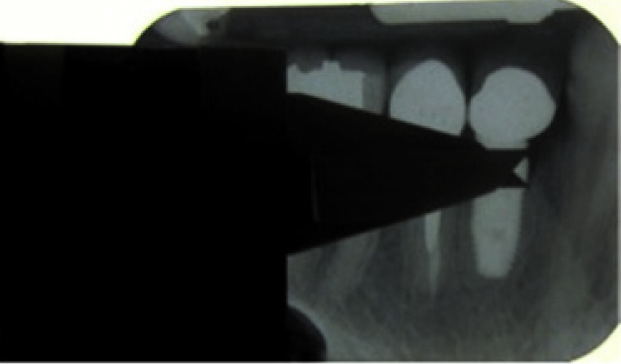
All measurements were done by a calibrated clinician blinded to the groups at baseline (loading time) and 3,6 and 12 months later and their changes were investigated.
Demographic features of the two groups were compared at baseline using the t-test for continuous variables or Pearson Chi square tests for discrete variables. Continuous data were presented as mean±SD for each clinical parameter. We used paired t-test to compare baseline data with 3,6 and 12 month data in each group. An independent-samples t-test was performed to compare clinical parameters between the two groups at baseline and 3,6 and 12 months. Data were analysed using repeated measures analysis of variance (ANOVA) and multiple comparisons were done using LSD method. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 16 statistical software.
Results
Study Population
In this study we assessed 48 patients (total of 48 implant procedures). Demographic data for both groups are listed in [Table/Fig-5].
Clinical Findings
Healing was uneventful in all 48 patients. At baseline, all patients presented with low levels of plaque accumulation (PI) and good gingival health(GI), and no significant difference between groups was noted (p<0.05).
Baseline Demographic Data for the Two Groups
| Data analysed | One stage group | Two stage group |
|---|
| Males | 10 | 12 |
| Females | 14 | 12 |
| Mean age ± SD (range) | 38±10.45 years (26 to 51) | 43±9.53 years (29 to 54) |
The baseline clinical parameters, including Probing Depth (PD), Keratinized Gingiva (KG), Mucosal Thickness (MT), PapillaIndex (PI) and hard tissue measurements were not significantly different between the groups [Table/Fig-6].
The soft and hard tissue parameters (mean ± SD).
| One stage group | Two stage group | |
|---|
| Parameter | Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 month | p-value |
|---|
| PD (mm) | 2.25±0.31 | 2.72±0.26 | 2.97±0.28 | 3.20±0.31 | 2.21±0.27 | 2.71 ±0.23 | 2.90±0.34 | 3.24±0.32 | 0.061 |
| KG (mm) | 2.87±0.27 | 3.32±0.31 | 3.40±0.41 | 3.34±0.32 | 3.07±0.29 | 3.58±0.34 | 3.76±0.42 | 3.59±0.38 | 0.069 |
| MT (mm) | 3.09±0.49 | 3.27±0.36 | 3.25±0.43 | 3.30±0.39 | 3.12±0.19 | 3.33±0.41 | 3.32±0.35 | 3.29±0.34 | 0.063 |
| PI | 2.48±0.37 | 2.14±0.32 | 2.03±0.27 | 2.10±0.29 | 2.35±0.42 | 2.14±0.31 | 1.98±0.26 | 1.93±0.23 | 0.138 |
| SC (mm) | 0.04±0.02 | 0.97±0.23 | 1.49±0.45 | 1.91 ±0.53 | 0.12±0.08 | 0.44±0.29 | 0.76±0.34 | 1.77±0.63 | 0.004 |
PD: Probing Depth, KG: Keratinized Gingiva, MT: Mucosal Thickness, PI: Papilla Index, SC:The distance between implant shoulder and alveolar crest
The changes of soft tissue parameters including PD, KG, MT and PI in the two groups during 3,6 and 12 months after loading had no significant difference either [Table/Fig-7].
The changes of soft and hard tissue (mean ± sd)
| Parameters | One stage | Two stage | |
|---|
| 3 months | 6 months | 12 months | 3 months | 6 months | 12 months | p-value |
|---|
| PD (mm) | +0.47±0.23 | +0.72±0.32 | +0.95±0.43 | +0.51±0.30 | +0.69±0.27 | +0.52±0.21 | 0.072 |
| KG (mm) | +0.45±0.34 | +0.53±0.31 | +0.47±0.38 | +1.25±0.27 | +1.50±0.39 | +1.39±0.40 | 0.069 |
| MT(mm) | +0.18±0.09 | +0.16±0.0.7 | +0.21±0.11 | +0.21±0.0.17 | +0.20±0.14 | +0.17±0.17 | 0.117 |
| PI | -0.34±0.12 | -0.45±0.23 | -0.38±0.43 | - 0.21±0.13 | -0.37±0.23 | -0.42±0.32 | 0.077 |
| SC (mm) | 0.93±0.45 | 1.45±0.58 | 1.87±0.76 | 0.32±0.21 | 0.74±0.43 | 1.65±0.59 | 0.004 |
PD: Probing Depth, KG: Keratinized Gingiva, MT: Mucosal Thickness, PI: Papilla Index, SC: The distance between implant shoulder and alveolar crest
The distance between implant shoulder and alveolar crest (SC) which represents the bone loss around implants (measured on standardized digital radiographs) 3 and 6 months after loading in the one stage group was significantly greater than the bone loss around the two stage group. But at 12 months loading there was no significant difference between the two groups [Table/Fig-7].
Discussion
The technique of surgery is important in success and survival of the implant. Some important factors in implant surgery include cooling the surgical site, the speed of drilling, primary stability of the implant, and perhaps one stage or two stage technique of the surgery. In early studies it was claimed that implants must be submerged during the healing period for successful tissue integration. Therefore, in many studies the survival rate and success rate of non submerged implants are evaluated. Weber et al., published the results of their five year evaluation study which suggested no relationship between bone loss and one stage insertion of implants [12]. In results of another study in 2010 cumulative survival rate of non submerged implants up to 16 years was 82.94% [13]. A multicenter five year prospective clinical trial the survival rate and success rate of non submerged implants was 99.4% and 99.5%, respectively. However, these studies did not compare submerged and non submerged implants [14]. In 2009 a Cochrane systematic review evaluated the failure of implants, bone loss and patient satisfaction in which only five studies were eligible for inclusion criteria of comparing the same dental implants placed as one-stage or two-stage and with a minimum of 6 months follow up after loading and it was reported that due to the small number of patients included in the studies, a definitive conclusion was not achievable [15].
In our study soft tissue characteristics such as mucosal thickness, keratinized gingiva, probing depth and papilla index, as well as hard tissue characteristics (distance of bone to shoulder of implants which represents the bone loss around implants) were assessed. The surgery conditions for all patients were similar and all of the surgeries were done by one operator.
One of the most important factors in implant surgery in the aesthetic zone is papilla reconstruction. Following extraction, bone loss ensues and the papilla recedes. Therefore, the reconstruction of papilla after implant placement is very critical. It has been reported that the factor that strongly affects the presence of papilla is the distance between contact point and bone crest and the greater this distance, the less the chance of forming the papilla [16]. Results of present study did not show any significant differences between the mean papilla index of loaded one stage or two stage implants at baseline and follow-ups; however, the papilla height is reduced during the follow- up period ,which can be contributed to peri implant bone loss and increasing the distance between implant contact to bone crest. Therefore, inserting healing abutment immediately after implant placement or later during the second surgery does not affect papilla reconstruction after inserting the implant crown.
The effect of submergence on keratinized tissue was also evaluated in the present study. The mean amount of keratinized tissue was not significantly different in submerged and non submerged implants. Therefore, the procedure of covering the implants with soft tissue for submerging them had no effects on decreasing the keratinized tissue. Moreover, the changes of keratinized tissue over time had no significant difference either. However, the method of exposing the implants during the second stage surgery is important in the amount of remained keratinized tissue and is not evaluated in the present study. There is no consensus on the importance of keratinized tissue around teeth and implants. Lang and Loe [17] suggested that at least 2 mm of keratinized gingiva and 1 mm of attached gingiva are necessary for healthy periodontium. The presence of keratinized tissue may have several advantages; color, contour and texture of gingiva depend on keratinized tissue and maintaining the oral hygiene may be easier. Keratinized gingiva has more hemidesmosomes and their fibers are perpendicular to the surface of the tooth. In addition in two stage surgery the keratinized tissue may decrease the probability of implant exposure during healing. But Wennstrom claimed that if there is good oral hygiene the amount of keratinized and attached gingiva is not very important for periodontal health [18].
One of the most important factors in implant success is preservation of peri-implant bone. The quality and quantity of bone affects implant osseointegration, survival and aesthetics. Some of the factors that have been expressed as causes of bone loss are microbial factors, overheating, micro-movement of abutment or prosthesis, functional/parafunctional forces and also the surgical protocols [19]. In this study the mean bone loss during 3 and 6 months following loading in one stage implants was more than in two stage implants (1.23 mm and 1.43 mm in one stage implants and 0.67 mm and 0.95 mm in two stage implants at 3 and 6 months; respectively). But after 12 months the difference between the two groups had no significant difference (1.34 mm in one stage implants versus 1.43 mm in two stage implants).The results of the present study are supported by those from several studies that have shown comparable bone loss in submerged and non submerged implants after loading. Cecchinato et al., [20,21] in 2 studies within a five year period showed that crestal bone loss is independent of implant submergence (mean marginal bone loss of 0.02 ± 0.38 mm and 0.17 ± 0.51 mm around 1-stage and 2-stage implants in the first year).The implants were distributed in different positions in maxilla and mandible with different densities which can affect marginal bone level. Petersson et al., [22] also reported similar results using Brånemark dental implants after 18 months (0.2 vs. 0.3 mm, respectively) and five years (1 mm in both groups).Their study had a split mouth design in anterior mandible site but they had a small sample size. A randomized clinical trial by Siadat et al., [23] reported that 3 months after implant placement, the 2-stage implants showed significantly more crestal bone loss and after 6 and 12 months of loading, bone level changes in both groups were not significantly different. They attributed this different bone loss after 3 months to temporizing the implants by relined dentures which can load one stage implants directly and two stage implants indirectly. The amount of reported bone loss is different in these studies; which can be contributed to different implant designs, bone density ordifferent radiographic techniques.
It is hoped that this research could contribute to solve the question about implant submergence. We can suggest that in situations where there are no need for simultaneous bone augmentation procedures, submergence of implants is not necessary and they can be inserted as one stage protocol which can simplify patient management because a second-stage exposure surgery is not necessary.
In this study, submerged and non submerged implants were inserted in posterior mandible area. Therefore, the effect of different bone density of various sites in the jaws is nearly eliminated. However, bone density in a special site is not similar in different patients and comparing these two surgery protocols in a study with split mouth design can minimize the confounding factors.
Conclusion
Within the limitations of this study, it is concluded that the technique of surgery,either one stage or two-stage,had no effect on soft tissue parameters.But with respect to hard tissues, it was observed that the bone loss around implants during 3 and 6 months following surgery in the one stage group was more than the two stage group; after 12 months there was no significant difference between the two groups.