Surgical resection is the mainstay of treatment and can cure patients with early-stage cancer. The survival rate of patients with advanced resectable gastric or gastroesophageal junction (GEJ) cancers, however, remains poor despite new treatment strategies, such as perioperative chemotherapy or adjuvant chemoradiation [4]. Objective response rates range from 10% to 30% for single-agent therapy and 30% to 60% for combination regimen [5] along with uncertainty regarding the choice of the chemotherapy regimen [6]. New rationally designed molecular targeted therapies are urgently needed which interfere with the signalling cascades involved in cell differentiation, proliferation and survival.
The HER2 protein (p185, HER2/neu, ErbB-2) is a 185-kDa transmembrane tyrosine kinase (TK) receptor and a member of the epidermal growth factor receptor (EGFR) family. HER2 is encoded by a gene located on chromosome 17q21. In carcinomas, HER2 acts as an oncogene, mainly because high-level amplification of the gene induces protein overexpression in the cellular membrane and subsequent acquisition of advantageous properties for a malignant cell [7]. Evaluated extensively in breast cancers, the role of HER 2 over-expression in gastric adenocarcinomas is being studied. With few reports from India [8–10] and none from southern part of India, we attempted to study the same at a medical college hospital based in Bengaluru.
Materials and Methods
This was an observational study including all histopathologically diagnosed adenocarcinomas of stomach and gastro esophageal junction (GEJ) which were diagnosed in the Department of Pathology, Kempegowda Institute of Medical Sciences, Bengaluru over a period of five years (from June 2009 to June 2014).
Relevant clinical details were collected from the patients’ and case files in prospective cases (29 cases), while available details were collected from the medical records in retrospective cases (31 cases). Radiological and endoscopy details were retrieved wherever possible. All gastrectomy/gastric biopsy specimens were fixed in 10% neutral buffered formalin. Tissues were processed, embedded in paraffin, sections were taken and stained with routine H&E. Histopathological diagnosis was made and adenocarcinomas were classified as intestinal / diffuse according to Lauren classification. Giemsa staining was performed for H. pylori status. Specimens which were tiny, not fixed were excluded from the study.
In retrospective cases (31 cases), slides were reviewed to confirm diagnosis. Respective paraffin blocks ( both from prospective and retrospective cases) were retrieved, 4 μ sections were taken and mounted on poly L lysine coated slides taking care to mount the tissue sections flat and wrinkle free as possible. The slides were placed in the oven at 600C for 30 min. Antigen retrieval was done using Citrate buffer at pH 5.5 to 6.0 in a domestic microwave at high power for 5 min. Endogenous peroxidase blocking was done with 3% hydrogen peroxide. Immnohistochemistry was done using Rabbit polyclonal HER 2 antibody in the dilution of 1:600 using Dako REALTM En Vision DetectionTM system, (Peroxidase /DAB+, K5007).
The Immunohistochemistry slides were scored by two pathologists individually according to scoring system by Hoffman et al., [Table/Fig-1]. All cases with score 3+ were considered positive.
Human epidermal growth factor receptor 2 (HER2) scoring criteria for gastric cancer
| Score | Surgical specimen-staining pattern | Biopsy specimen-staining pattern | HER2 over expression assessment |
|---|
| 0 | No reactivity or membranous reactivity in < 10%of Tumour cells | No reactivity or no membranous reactivity in any Tumour cell | Negative |
| 1+ | Faint/barely perceptible membranous reactivity in ≥10% of Tumour cells; cells are reactive only in part of their membrane | Tumour cell cluster with a faint/barely perceptible membranous reactivity irrespective of percentage of Tumour cells stained | Negative |
| 2+ | Weak to moderate complete, basolateral, or lateral membranous reactivity in ≥10% of Tumour cells | Tumour cell cluster with a weak to moderate complete, basolateral, or lateral membranous reactivity irrespective of percentage of Tumour cells stained | Equivocal |
| 3+ | Strong complete, basolateral, or lateral membranous reactivity in ≥10% of Tumour cells | Tumour cell cluster with a strong complete, basolateral, or lateral membranous reactivity irrespective of percentage of Tumour cells stained | Positive |
Statistical Analysis
Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented on Mean + SD (Min-Max) and results on categorical measurements are presented in Number (%). Significance is assessed at 5 % level of significance. The following assumptions on data was made,
Assumptions
1. Dependent variables should be normally distributed,
2. Samples drawn from the population should be random, Cases of the samples should be independent.
Student t-test was used to find the significance of study parameters on continuous scale between two groups on metric parameters. Chi-square/ Fisher Exact test has been used to find the significance of study parameters on categorical scale between two or more groups.
Statistics: + Suggestive significance (p-value: 0.05<p<0.10)
* Moderately significant ( p-value:0.01<p ≤ 0.05)
** Strongly significant (p-value: p≤0.01)
The Statistical software, SAS 9.2, SPSS 15.0, Stata 10.1, Med Calc 9.0.1,Systat 12.0 and R environment ver.2.11.1 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc.
Results and Observation
The study included 60 proven cases of adenocarcinomas of which, 44 were biopsies and 16 were gastrectomy specimens. Age of patients ranged from 31-85 y. Majority of patients belong to the age group of 61-70 y. Mean age of the patients was 65.65 y with males accounting to 60% [Table/Fig-2]. Most of the tumours were located in antrum (76.7%) followed by GEJ. According to Lauren classification, 81.7 % of Tumours were of intestinal type and the rest were of diffuse type (18.3%). Moderately differentiated Tumours were the predominant (66.7%) type followed by poorly differentiated (18.3%) and well differentiated type (15%). (p=0.013* Significant, Chi-Square test).
Gender distribution in the study group
| Gender | Total number of patients | HER2 |
|---|
| 0 | 1+ | 2+ | 3+ |
|---|
| Female | 24 | 12(50%) | 4(16.7%) | 3(12.5%) | 5(20.8%) |
| Male | 36 | 25(69.4%) | 0(0%) | 0(0%) | 11(30.6%) |
| Total | 60 | 37(61.7%) | 4(6.7%) | 3(5%) | 16(26.7%) |
P=0.006**, significant, Fisher Exact test
HER2 scores of patients studied: Majority of patients was of score 0 (61.7%) while 26.7% of patients showed a score of 3+suggesting HER 2 positivity [Table/Fig-3,4,5]. HER2 positivity was more in gastric biopsies (31.8%) as opposed to 12.5% in gastrectomies [Table/Fig-6]. A score of 2+ i.e., equivocal was seen in 5% of cases and 6.7% of cases were of score 1+ [Table/Fig-7]. HER2 positivity was found more in the age group of 31-40 y (2 out of 3 cases studied were of score 3+ and one case was equivocal). (p=0.253, NS, Fisher Exact test). HER2 positivity was found more in males (30.6%). Intestinal type appeared to express HER2 in relatively lower percentage of Tumours (32.7 %). Adenocarcinomas of gastric origin expressed HER 2 in 22.4% when compared to the GEJ Tumours (45.5%). None of the diffuse type showed HER2 positivity [Table/Fig-8]. HER2 positivity was found more frequently in moderately differentiated Tumours (37.5%), followed by 11.1% in well-differentiated Tumours [Table/Fig-9]. None of the poorly differentiated Tumours expressed HER2 positivity [Table/Fig-10]. We observed in a minority of cases intense cytoplasmic positivity in the Tumour cells (6/60cases). No correlation was found between HER2 positivity and pathological stage (pTNM). In our study most patients were of pathological stage T2 (87.5%) while 1 case each (6.25% each) was of stage T3 and T4. Regarding the pathologic N stage, only 3 cases (18.75%) were of N 0 stage, rest were of N 1 stage (81.25%). Distant metastasis was not documented in any of the cases.
Moderately differentiated adenocarcinoma showing strong membranous HER2 positivity (score 3+). Case no : 1 (400x, IHC)
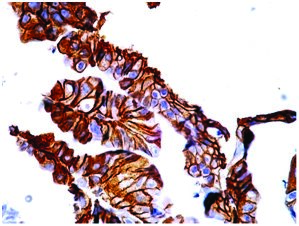
Well-differentiated adenocarcinoma (gastrectomy ) showing strong lateral wall staining on HER2 (score 3+). Case no : 55 (400x, IHC)
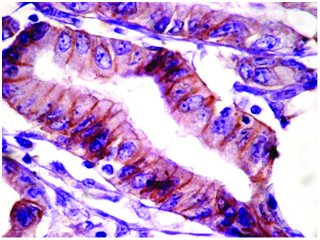
Signet ring cell carcinoma showing absence of HER2 staining (score 0). Case no : 52 (400x, IHC)
HER2 scores of patients studied
| HER2 | Gastric biopsies | Gastrectomies | Total |
|---|
| 0 | 24(54.5%) | 13(81.3%) | 37(61.7%) |
| 1+ | 3(6.8%) | 1(6.3%) | 4(6.7%) |
| 2+ | 3(6.8%) | 0(0%) | 3(5%) |
| 3+ | 14(31.8%) | 2(12.5%) | 16(26.7%) |
| Total | 44(100%) | 16(100%) | 60(100%) |
Correlation of HER 2 with Lauren Classification
| Laurens Clasification | Total number of patients | HER2 |
|---|
| 0 | 1+ | 2+ | 3+ |
|---|
| Diffuse | 11 | 10(90.9%) | 0(0%) | 1(9.1%) | 0(0%) |
| Intestinal | 49 | 27(55.1%) | 4(8.2%) | 2(4.1%) | 16(32.7%) |
| Total | 60 | 37(61.7%) | 4(6.7%) | 3(5%) | 16(26.7%) |
P=0.054+, significant , Fisher Exact test
Correlation of histological grade with HER 2
| Grade | Total number of patients | HER2 |
|---|
| 0 | 1+ | 2+ | 3+ |
|---|
| Poorly | 11 | 10(90.9%) | 0(0%) | 1(9.1%) | 0(0%) |
| Moderately | 40 | 19(47.5%) | 4(10%) | 2(5%) | 15(37.5%) |
| Well Differentiated | 9 | 8(88.9%) | 0(0%) | 0(0%) | 1(11.1%) |
| Total | 60 | 37(61.7%) | 4(6.7%) | 3(5%) | 16(26.7%) |
P=0.042*, Significant , Fisher Exact test
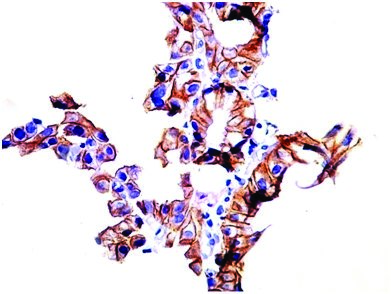
Moderately differentiated adenocarcinoma (GEJ), showing strong membranous HER2 positivity (score 3+). Case no : 16 (400x, IHC)
Comparison of Her 2 expression in gastric cancers reported by various authors with our study [7–10,25]
| Authors (year) | No of cases studied | Geographic zone | HER2 expression by IHC in % |
|---|
| Yano et al., [7] | n= 200 | Japan | 23 |
| Gravalos et al., [7] | n= 166 | Europe | 13 |
| Park et al., [25] | n= 182 | Korea | 16 |
| Lordick et al., [7] | n= 1527 | Europe, Asia, Latin America | 22 |
| Shekharan A et al., [8] | n= 52 | India | 44.2 |
| Prachi S Patil et al., [9] | n= 43 | India | 7 |
| Mallika Tewari et al., [10] | n= 70 | North India (Varanasi) | 21.4 |
| Our Study (2014) | n= 60 | India | 26.7 |
Discussion
The first cases of possible gastric cancer were reported in the Ebers Papyrus, written in 1600 BC. Benign and malignant gastric ulcers were only described later by J. Cruveilhier in 1835 demystifying the death of Napolean Bonaparte in 1821 who probably had an extensive scirrhous carcinoma of the stomach, complicated by partial gastric obstruction [11].
The incidence of gastric cancer varies in different parts of the world with highest incidence rates documented in Eastern Asia, South America, Eastern Europe [12,13] (fifth most common cancer in Europe with 159 900 new cases and 118 200 deaths in 2006) [14]. Linxian, China is known to have one of the highest rates of oesophageal/gastric cardia cancer in the world [15]. North America and Africa show the lowest recorded rates [12,13]. In India gastric cancer is the fifth most common cancer among males [16] (7th in Karnataka) [17] and seventh most common cancer among females [16] (8th in Karnataka [17]). According to the population based cancer registry at Kidwai Memorial Institute of oncology, under the national cancer registry program of ICMR, which covers the resident population of Bangalore urban Agglomeration, the most predominant site of cancer constituting 9% of total cancers among males is stomach cancer [17]. Improved food hygiene, sanitation, and food preservation techniques have lead to a decline in the incidence of gastric cancer all over the world. But in certain parts of India, gastric cancer in South Indian males has been reported to be more common and occurring a decade before their North Indian counterparts. Differences in some dietary pattern and use of tobacco and alcohol have been considered as potential risk factors [18].
Many gastric cancer patients present with advanced stage disease, and the prognosis remains poor. Improvements in the treatment of gastric cancer, including combination chemotherapy, have resulted in improved overall survival versus single-agent chemotherapy alone. Additional therapy aimed at specific targets in cancer has shown a survival benefit in certain Tumours. One of these cellular targets, human epidermal growth factor receptor 2 (HER2/neu) protein, a 185-kDa transmembrane tyrosine kinase receptor, is associated with Tumour proliferation, migration, and differentiation.
The overexpression of HER2/neu on Tumour cells versus normal cells allows selective targeting of malignant cells with anti-HER2/neu therapy. HER2/neu overexpression has been identified in a variety of neoplasms but has been most widely studied in breast cancer. Approximately 25% to 30% of breast cancers overexpress HER2/neu. HER2/neu-positive status is associated with more aggressive disease and is an important predictive factor of response to therapy with trastuzumab [19].
Recently published data, from the randomized, prospective phase III clinical trial ToGA provided first documentation of the clinical benefit of trastuzumab when used in combination with chemotherapy in the setting of advanced gastric and gastroesophageal junction (GEJ) cancer. Median overall survival was 13.8 months in trastuzumab arm compared to11.1 months in the chemotherapy only arm. As a result of the survival benefit, laboratories are facing increasing demand for HER2 tests to determine patient eligibility for targeted therapy [20] In addition to trastuzumab several ongoing trials are testing the efficacy of other targeted agents in gastric cancer. Lapatinib is a TK inhibitor that targets both HER2 and EGFR and can be administered orally. Novel antibodies like Pertuzumab which is a dimerisation inhibitor, in combination with trastuzumab has been evaluated in trastuzumab resistant HER2 positive metastatic breast cancer. This may be worthy of investigation in early gastric cancers. Bevacizumab a monoclonal VEGF antibody has been investigated in locally advanced and metastatic gastroesophageal cancers [21].
Data reported in literature on HER2 positivity rates in gastric cancers vary from about 7 to 43 % [Table/Fig-11]. In our study, HER2 positivity was observed in 26.7% of cases. HER2 over expression was more in gastric biopsies (31.8%), whereas only 12.5 % cases of resected specimens showed positivity. Higher rates of HER2 positivity in biopsies may have been due to larger sample size of biopsies (n=44) when compared to gastrectomy (n=16). Another reason may be better fixation of biopsy specimens as suggested by Ruschoff J et al., [22].
Comparison of HER2 expression in gastric cancers reported by various authors based on tumor location and histological type with our study [7,10,23]
| Author | Histologic type | Localisation |
|---|
| Intestinal | Diffuse | GEJ | Gastric |
|---|
| Tanner et al., [7] | 21.5 (n=65) | 2 (n=46) | 24 (n=76) | 12 (n=115) |
| Gravalos et al., [7] | 16 | 7 | 25 | 9.5 |
| Lordick et al., [7] | 34 | 6 | 32 | 18 |
| Shan et al., [23] | 16.8 (n=650) | 2.3(n=564) | 45.5 | 22.4 |
| Mallika Tiwari et al., [10] | 45 (n=11) | 12 (n=44) | — | — |
| Our Study | 32.7(n=49) | 0 (n=11) | 45.5 | 22.4 |
Our study results matches with the study done by Yano et al., and Lordick et al., and also with TOGA trial which reported HER2 positivity of 22.1% overall and 10.4% in resected samples. In a study by Ling, Jianming et al., [23] out of 1463 patients, HER2 over expression was noted in 9.8% while 14.4% and 75.8% were equivocal and negative respectively. This discrepancy in results with our study may be attributed to the use of rabbit monoclonal primary antibody in their study whereas we used polyclonal antibody which target more epitopes [24].
Similar to Ling Shan et al., [23] we did not find statistically significant correlation of HER 2 positivity with age, sex and pTNM staging. However, in the study we had 3 cases in the age group of 31-40 yrs and 2 (66.7%) showed score of 3+ suggesting HER 2 positivity and the remaining one case showed score of 2+ (equivocal). Both these cases were adenocarinomas of moderately differentiated type but still were HER 2 positive. This is a significant observation as we are of the opinion that if survival benefit or significant disease free interval is offered by anti HER 2 treatment, it would be of great help in younger age group who are at their productive best.
In contrast to previous studies, we found a statistically significant correlation of HER2 with male gender (30.6%, p = 0.006). This may be attributed to greater number of male patients in our study as gastric adenocarcinomas are more common in males.
Our study showed 45.5% HER2 positivity in GEJ Tumours Vs 22.4% in primary gastric Tumours. This is in concordance with most reports [Table/Fig-11]. Statistically significant correlation was found between HER2 positivity and intestinal type of cancer (32.7%, p = 0.054). The reasons for the selective overexpression of HER2 in intestinal type of gastric carcinomas are complex. The association of HER2 with a specific histologic Tumour type suggests that intestinal and diffuse types develop along different molecular pathways. In gastric cancer, HER2 gene amplification associates inversely with E-cadherin mutations. E-cadherin mutations are typical for diffuse gastric cancers but rare in intestinal gastric cancers [7]. None of the diffuse cases were positive for HER2. Though review of literature shows decreased percentage of diffuse type of gastric cancers with HER2 overexpression, none of the studies have recorded a total absence of HER2 expression. We attribute this to the smaller number of diffuse type of gastric cancers in our study i.e. 11/60. As the gastric cancer epidemiology is different within India, i.e., North and South India, is geographic variation a reason for negative HER2 expression in diffuse type? An exclusive study with large number of diffuse type cancers with regional comparison may give an answer. No correlation was found between HER2 positivity and pathological stage in most of the earlier studies [26–28].
HER2 positivity and Tumour grade showed significant statistical correlation (p = 0.042), in concordance with that of Shan et al., [23], Chao He et al., [29] and Laura Tafe et al., [30] who observed higher rates of HER2 positivity in well and moderately differentiated carcinomas than poorly differentiated cancers. Study group consisted of 66.7% of moderately differentiated carcinomas and hence the frequency of HER2 positivity was also more. Another reason could be that as 49/60 cases were biopsy specimens where a tendency to grade Tumours to the in -between moderate grade due to limited tumour sample, may be contributory. Most authors [23,30,31] have also demonstrated increasing HER2 expression in moderately differentiated Tumours. A multicenter trial with equal number of cases in each category (well, moderate and poorly differentiated) would evaluate whether moderate differentiation is an independent risk factor?
Similar to many authors [26–29] there was no correlation between HER2 positivity and H. pylori status. The progression in H. pylori associated gastric cancers begins with H. pylori causing intestinal metaplasia moving on to dysplasia and last to malignant transformation [32]. During sampling for diagnosis of malignancy, we always sample the Tumour part, which no longer hosts the H. pylori, contributing to the negative H. pylori status. More so in the case of biopsies, we are studying only the Tumourous part and the possibility of detecting H. pylori is remote. Probably if we had studied an area away from Tumour in case of gastrectomies, we might have been able to detect the presence or absence of H. pylori association. As majority of gastrectomies were retrospective, we were unable to study the mucosa away from Tumourous area.
One of the drawbacks of our study is that we were not able to confirm IHC 1+ and 2+ cases by FISH. Studies have shown excellent IHC-FISH concordance (>95%) in IHC 0 and IHC 3+ cases, suggesting that these IHC scores may not require routine FISH confirmation. Reliable separation of IHC 1+ and IHC 2+ patterns is challenging in small biopsy specimens, due to crush and edge artifacts [30].
Preliminary data from TOGA trial showed that patients with amplified Tumours without overexpression (IHC 0 and 1+ cases) did not show substantial overall survival benefit from trastuzumab. Still we may have missed some of the IHC 2+ cases which would have shown HER2 amplification by FISH [21]. Another pitfall of IHC that we noticed was that normal foveolar epithelium showed non specific cytoplasmic staining. Though there was clear distinction between normal epithelium and Tumour cells and this did not interfere with our interpretation, one need to be careful during interpretation of HER2 staining. Intense cytoplasmic positivity (probably related to cytoplasmic mucin) in the Tumour cells though considered non -specific in the study, needed FISH confirmation because strong cytoplasmic staining can mask membranous positivity [30].
Conclusion
An accurate assessment of HER2 expression in gastric cancer patients is of importance and utility in the optimal selection of patients for Trastusumab (Herceptin) therapy. Our study found an HER2 overexpression of 22.4% in gastric cancers similar to most studies in India and rest of the world. We found a statistically significant correlation of HER2 overexpression with male gender, intestinal-type and moderately differentiated gastric cancers suggesting that these may be the candidates for targeted therapy using Herceptin. Additional studies are needed to explore the role of HER2 as an independent prognostic factor. Also diffuse type of gastric cancers not expressing HER2 needs to be studied, to confirm any existing geographic variation .Though Herceptin is approved for advanced gastric and GEJ cancers, role of herceptin in adjuvant / neo-adjuvant setting in early stages needs to be evaluated with newer agents like Pertuzumab, Bevacizumab, especially in young patients.
P=0.006**, significant, Fisher Exact test
P=0.054+, significant , Fisher Exact test
P=0.042*, Significant , Fisher Exact test