Introduction
Debridement of the root canal system is essential for endodontic success. Irrigation is a vital part of root canal debridement. It is impossible to shape and clean the root canal completely because of the intricate nature of root canal anatomy. Even with the use of rotary instrumentation, the nickel-titanium instruments currently available only act on the central body of the canal, leaving canal fins, isthmi, and cul-de-sacs untouched after completion of the preparation. These areas might harbor tissue debris, microbes, and there by-products, which might prevent close adaptation of the obturation material and result in persistent periradicular inflammation [1].
An ideal irrigant should reduce instrument friction during preparation, Facilitate dentin removal, Dissolve inorganic and organic tissue, Penetrate to canal periphery, Kill bacteria and yeasts and least irritating to the periapical tissues. However, there is no one unique irrigant that can meet al.,l these requirements, even with the use of methods such as lowering the pH, increasing the temperature, as well as addition of surfactants to increase the wetting efficacy of the irrigant. More importantly, these irrigants must be brought into direct contact with the entire canal wall surfaces for effective action, particularly for the apical portions of small root canals [2].
To accomplish these objectives, there must be an effective delivery system to working length. An improved delivery system for root canal irrigation is highly desirable. Such a delivery system must have adequate flow and volume of irrigant to working length to be effective in debriding the canal system without forcing the solution into periradicular tissues [2]. In selecting an irrigant and technique, consideration must be given to their efficacy and safety.
Today’s irrigation armamentarium presents a diverse variety of tools [Table/Fig-1] and techniques that can assist the practitioner in reducing bacteria and debris within the canal system. However, currently there is no universally accepted standard irrigation technique. Since most research comparing the efficacy of different irrigation techniques are in vitro studies with low levels of clinical evidence, caution is advised when considering the purchase of these devices [3].
A). Manual Agitation Techniques
Syringe Irrigation with Needles/Cannulas
Conventional irrigation with syringes has been advocated as an efficient method of irrigant delivery before the advent of passive ultrasonic activation. This technique is still widely accepted by both general practitioners and endodontists. The technique involves dispensing of an irrigant into a canal through needles/cannulas of variable gauges, either passively or with agitation. The latter is achieved by moving the needle up and down the canal space. Irrigation tip gauge and tip design can have a significant impact on the irrigation flow pattern, flow velocity, depth of penetration, and pressure on the walls and apex of the canal [2]. Irrigation tip gauge will largely determine how deep an irrigant can penetrate into the canal. A 21-gauge tip can reach the apex of an ISO size 80 canal, a 23-gauge tip can reach a size 50, a 25-gauge tip can reach a size 35 canal and a 30-gauge tip can reach the apex of a size 25 canal. 27 gauge needle is the preferred needle tip size for routine endodontic procedures. Several studies have shown that the irrigant has only a limited effect beyond the tip of the needle because of the dead-water zone or sometimes air bubbles in the apical root canal, which prevent apical penetration of the solution [4,5].
Needle-tip design
The smaller needles allow delivery of the irrigant close to the apex, this is not without safety concerns. Several modifications of the needle-tip design [Table/Fig-2a&Table/Fig-2b] have been introduced in recent years to facilitate effectiveness and minimize safety risks. Open-ended tips express irrigant out the end towards the apex and consequently increase the apical pressure within the canal. Closed-ended irrigant tips are side-vented and thus create more pressure on the walls of the root canal and improve the hydrodynamic activation of an irrigant and reduce the chance of apical extrusion. This allows the irrigant to reflux and causes more debris to be displaced coronally, while avoiding the inadvertent expression of the irrigant into periapical tissues [6].
One of the advantages of syringe irrigation is that it allows comparatively easy control of the depth of needle penetration within the canal and the volume of irrigant that is flushed through the canal [7,8].
Syringes
Plastic syringes of different sizes (1–20 mL) are most commonly used for irrigation [Table/Fig-3]. Although large-volume syringes potentially allow some time-savings, they are more difficult to control for pressure and accidents may happen. Therefore, to maximize safety and control, use of 1- to 5-mL syringes is recommended instead of the larger ones. All syringes for endodontic irrigation must have a Luer-Lok design. Because of the chemical reactions between many irrigants, separate syringes should be used for each solution [9].
Brushes
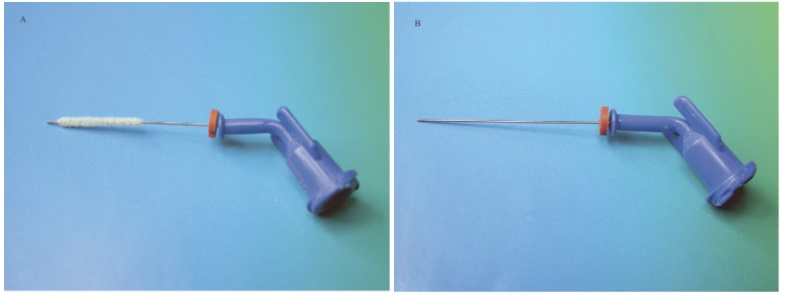
Strictly speaking, brushes are not directly used for delivering an irrigant into the canal spaces. They are adjuncts that have been designed for debridement of the canal walls or agitation of root canal irrigant. They might also be indirectly involved with the transfer of irrigants within the canal spaces. Recently, a 30-gauge irrigation needle covered with a brush (NaviTip FX; Ultradent Products Inc, South Jordan, UT) was introduced commercially [Table/Fig-4a,b]. A recent study reported improved cleanliness of the coronal third of instrumented root canal walls irrigated and agitated with the NaviTip FX needle over the brushless type of NaviTip needle. However, friction created between the brush bristles and the canal irregularities might result in the dislodgement of the radiolucent bristles in the canals that are not easily recognized by clinicians, even with the use of a surgical microscope [9,10].
During the early 1990s, similar findings indicating improved canal debridement with the use of canal brushes were reported by Keir et al., They used the Endobrush in an active brushing and rotary motion. The Endobrush (C&S Microinstruments Ltd, Markham, Ontario, Canada) is a spiral brush designed for endodontic use that consists of nylon bristles set in twisted wires with an attached handle and has a relatively constant diameter along the entire length. However, the Endobrush could not be used to full working length because of its size, which might lead to packing of debris into the apical section of the canal after brushing [8].
Vapor Lock Effect
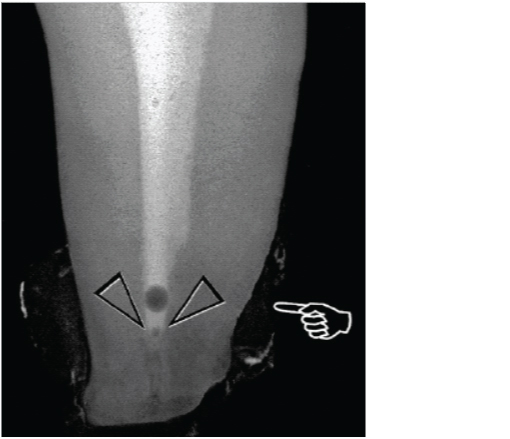
Air entrapment by an advancing liquid front in closed-end microchannels is a well-recognized physical phenomenon and has been referred to as the vapour lock effect [Table/Fig-5] in the endodontic literature. The ability of a liquid to penetrate these closed-end channels is dependent on the contact angle of the liquid and the depth and size of the channel. Because endodontic irrigation is performed within a time frame of minutes instead of hours or days, air entrapment in the apical portion of the canal might preclude this region from contact or disinfection by the irrigant [10-13].
Senia et al., demonstrated that NaOCl did not extend any closer than 3 mm from working length, even after root apex was enlarged to a size 30 [13,14]. This might be attributed to the fact that NaOCl reacts with organic material in the root canal and quickly forms micro gas bubbles at the apical termination that coalesce into an apical vapour lock with subsequent instrumentation. Because the apical vapour lock cannot be displaced within a clinically relevant time frame through simple mechanical actions, it prevents further irrigants from flowing into the apical region [2,15].
More importantly, acoustic microstreaming and cavitation can only occur in a liquid phase. Therefore, once a sonic or ultrasonically activated tip leaves the irrigant enters the apical vapor lock, acoustic microstreaming and/or cavitation becomes physically impossible [2,16].
A simple method to disrupt the vapor lock might be achieved via the use of a hand-activated well-fitting root filling material (e.g., a size 40, 0.06 taper gutta-percha point) that is introduced to working length after instrumentation with the corresponding nickel-titanium rotary instrument (i.e., size 40, 0.06 taper). This method, although cumbersome, eliminates the vapour lock because the space previously occupied by air is replaced by the root filling material, carrying with it a film of irrigant to the working length [2].
Manual-Dynamic Irrigation
An irrigant must be in direct contact with the canal walls for effective action. However, it is often difficult for the irrigant to reach the apical portion of the canal because of the so-called vapor lock effect. Research has shown that gently moving well-fitting gutta-percha master cone up and down in short 2 to 3 mm strokes (manualdynamic irrigation) within an instrumented canal can produce an effective hydrodynamic effect and significantly improve the displacement and exchange of any given reagent. This was recently confirmed by the studies of McGill et al., and Huang et al.,. These studies demonstrated that manual-dynamic irrigation was significantly more effective than an automated-dynamic irrigation system (RinsEndo; Duerr Dental Co, Bietigheim-Bissingen, Germany) and static irrigation [17].
Factors Affecting Manual Dynamic Irrigation
Several factors could have contributed to the positive results of manual dynamic irrigation: (1) the push-pull motion of a well fitting gutta-percha point in the canal might generate higher intracanal pressure changes during pushing movements, leading to more effective delivery of irrigant to the "untouched" canal surfaces; (2) the frequency of push-pull motion of the gutta-percha point (3.3 Hz, 100 strokes per 30 seconds) is higher than the frequency (1.6 Hz) of positive-negative hydrodynamic pressure generated by RinsEndo, possibly generating more turbulence in the canal; and (3) the push-pull motion of the gutta-percha point probably acts by physically displacing, folding, and cutting of fluid under ‘‘viscously-dominated flow’’ in the root canal system. The latter probably allows better mixing of the fresh unreacted solution with the spent, reacted irrigant.
Although manual-dynamic irrigation has been advocated as a method of canal irrigation as a result of its simplicity and cost-effectiveness, the laborious nature of this hand-activated procedure still hinders its application in routine clinical practice. Therefore, there are a number of automated devices designed for agitation of root canal irrigants that are either commercially available or under production by manufacturers [17].
B). Mechanical Agitation Techniques
1. Rotary Brush
Both Ruddle brush and Canal Brush both fit in this category.
I. A rotary handpiece–attached microbrush has been used to facilitate debris and smear layer removal from instrumented root canals [18]. The brush includes a shaft and a tapered brush section. The latter has multiple bristles extending radially from a central wire core. During the debridement phase, the microbrush rotates at about 300 rpm, causing the bristles to deform into the irregularities of the preparation. This helps to displace residual debris out of the canal in a coronal direction. However, this product has not been commercially available since the patent was approved in 2001.
II. Canal Brush is another endodontic microbrush that has recently been made commercially available. This highly flexible microbrush is molded entirely from polypropylene and might be used manually with a rotary action. Weise et al., showed that debris was effectively removed from simulated canal extensions and irregularities with the use of the small and flexible CanalBrush with an irrigant [19].
2. Continuous Irrigation During Rotary Irrigation
The Quantec-E irrigation system (SybronEndo, Orange, CA) is a self-contained fluid delivery unit that is attached to the Quantec-E Endo System. It uses a pump console, two irrigation reservoirs, and tubing to provide continuous irrigation during rotary instrumentation (Walters et al., 2002) [10].
3. Sonic Irrigation
Sonic instruments for endodontics were first reported by Tronstad et al., [19]. Sonic irrigation operates at a lower frequency (1–6 kHz) and produces smaller shear stresses than ultrasonic irrigation Ahmed cleanlinesset al., [20]. The EndoActivator is one form of the sonic irrigation that uses noncutting polymer tips to quickly and vigorously agitate irrigant solutions during treatment. A study has shown this method to be effective [Table/Fig-6].
Vibringe
Vibringe (Vibringe BV, Amsterdam, The Netherlands) is a new sonic irrigation system that combines battery-driven vibrations (9000 cpm) with manually operated irrigation of the root canal. Vibringe uses the traditional type of syringe/needle delivery but adds sonic vibration. No studies can be found on Medline [10].
4. Ultrasonic Irrigation
Ultrasonics is another group of instruments that can be used for irrigation in the ultrasonics and subsonic handpieces. Ultrasonic handpieces pass sound waves to an endodontic file and cause it to vibrate at ~25,000 vibration/s. It cuts dentin as well as causes acoustic streaming of the irrigant (Martin and Cunningham). It was also found that debris dislodgment from canal walls occurs through cavitation occurring within the irrigating solution. The dental literature has described two types of ultrasonic irrigation. The first one is a combination of simultaneous ultrasonic instrumentation and irrigation (UI). The second one operates without simultaneous instrumentation and is referred to as passive ultrasonic irrigation (PUI).
PUI is more effective than syringe needle irrigation at removing pulpal tissue remnants and dentine debris. This may be due to the much higher velocity and volume of irrigant flow that are created in the canal during ultrasonic irrigation. Ultrasonics can effectively clean debris and bacteria from the root canal system, but cannot effectively get through the apical vapor lock [31-33].
Positive pressure versus apical negative pressure
There are two apparently dilemmatic phenomena associated with conventional syringe needle delivery of irrigants. It is desirable for the irrigants to be in direct contact with canal walls for effective debris debridement and smear layer removal. Yet, it is difficult for these irrigants to reach the apical portions of the canals because of air entrapment, when the needle tips are placed too far away from the apical end of the canals. Conversely, if the needle tips are positioned too close to the apical foramen, there is an increased possibility of irrigant extrusion from the foramen that might result in severe iatrogenic damage to the periapical tissues [2]. Concomitant irrigant delivery and aspiration via the use of pressure alternation devices provide a plausible solution to this problem.
Early Experimental Protocols
The first experimental use of a pressure alternation irrigation technique was the non-instrumentation technology (NIT) invented by Lussi et al., [34]. This technique did not enlarge root canals because there was no mechanical instrumentation of the canal walls. Although, NIT was unique and successful in vitro, the technique was not considered safe in invivo animal studies and did not proceed to human clinical trials.
Another experimental pressure alternation irrigation system was introduced by Fukumoto et al., [35]. This system comprised an injection needle (external diameter, 0.41 mm; internal diameter, 0.19 mm; Nipro Co, Osaka, Japan) and an aspiration needle (external diameter, 0.55 mm; internal diameter, 0.30 mm; Terumo Co, Tokyo, Japan) connected to an apex locator (Root ZX; J Morita USA, Inc, Irvine, CA). The aspiration pressure of the unit was maintained at –20 kPa. The device was evaluated by using different placement positions of the injection needle and the aspiration needle for the efficacy of smear layer removal from the apical third of the canal walls and the frequency of extrusion of NaOCl from the apical foramen. The most reliable results were achieved when NaOCl was introduced by using a coronally placed injection needle and aspirated via placement of the aspiration needle at 2mm from the apex. Of particular importance was that when the aspiration needle was placed either 2 or 3 mm from the apical end of the root, the Root ZX readings registered a value of 0.5, indicating that the irrigant had reached the instrumented end of the apical delta [2,35].
5. Pressure Alternation Devices
The RinsEndo irrigation system and the EndoVac irrigation system are examples of negative-pressure irrigation.
I. The RinsEndo irrigation system (RinsEndo, Co. Duerr- Dental, Bittigheim-Bissingen, Germany) irrigates the canal by using pressure-suction technology. It is composed of a handpiece, a cannula with a 7-mm-long exit aperture, and a syringe carrying irrigant [36].
II. The EndoVac system is regarded as an apical negative pressure irrigation system composed of three basic components: a Master Delivery Tip (MDT), the Macrocannula, and the Microcannula. The MDT delivers irrigant to the pulp chamber and evacuates the irrigant concomitantly. Both the macrocannula and microcannula are connected via tubing to a syringe of irrigant and the highspeed suction of a dental unit. The Macrocannula is made of plastic flexible polypropylene with an open end of 0.55 mm in diameter, an internal diameter of 0.35 mm, and a 0.02 taper, used to suction irrigants up to the middle segment of the canal. Lastly, the Microcannula threeis made of stainless steel and has 12 microscopic holes disposed in four rows of three holes, laterally positioned at the apical 1 mm of the cannula. Each hole is 0.1 mm in diameter, the first one in the row is located 0.37 mm from the tip of the microcannula, and the distance between holes is 0.2 mm. The microcannula has a closed end with external diameter of 0.32 mm can be used in canals that are enlarged to size 35 or larger, and should be taken to the working length (WL) to aspirate irrigants and debris. During irrigation, the MDT delivers irrigant to the pulp chamber and siphons off the excess irrigant to prevent overflow. The cannula in the canal simultaneously exerts negative pressure that pulls the irrigant from its fresh supply in the chamber by the MDT, down the canal to the tip of the cannula, into the cannula, and out through the suction hose. Thus, a constant flow of fresh irrigant is being delivered by negative pressure to working length.
Nielsen and Baumgartner [37] compared the efficacy of the EndoVac system and needle irrigation to debride the apical 3 mm of a root canal. No significant difference between the two irrigation techniques was noted at the apical 3 mm level. But at 1 mm apical level, the EndoVac system significantly resulted in less remaining debris. The Endovac irrigation system was also shown to achieve better microbial control than the traditional irrigation delivery system (Hockett et al.,. Miller and Baumgartner) [38]. Another in vitro study indicated that EndoVac left significantly less debris behind than the conventional 30-gauge needle irrigation methods (Shin et al.,) [39].
In contrast, two very recent studies showed the opposite results. The first by Townsend and Maki who conducted a study on plastic simulated canals, found that the EndoVac irrigation system was significantly less effective in removing bacteria when compared with ultrasonic irrigation [40]. Another study by Brito et al.,) [42] found no significant difference in bacterial reduction efficiency between the Endovac system, the NaviTip needle and the EndoActivator sonic system. Various studies related Endovac are listed in [Table/Fig-7].
Various irrigation techniques
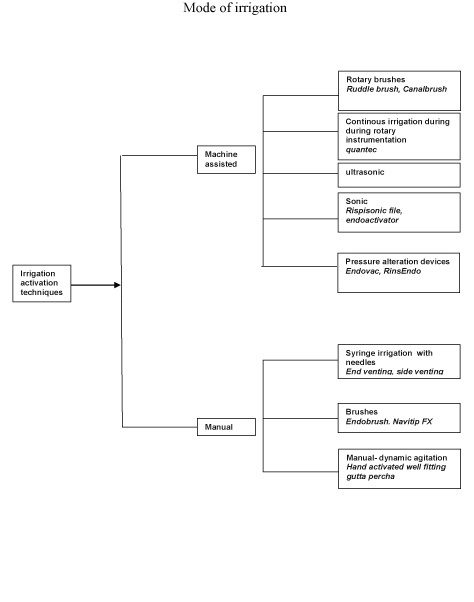
(A-C) Open-ended needles: (A) flat (NaviTip; Ultradent, South Jordan, UT), (B) beveled (PrecisionGlide Needle; Becton Dickinson & Co, Franklin Lakes, NJ), and (C) notched (Appli- Vac Irrigating Needle Tip; Vista Dental, Racine, WI). (D-F) Closed-ended needles: (D) side vented (KerrHawe Irrigation Probe; KerrHawe SA, Bioggio, Switzerland), (E) double side vented (Endo-Irrigation Needle; Transcodent, Neumu¨ nster, Germany), and (F) multivented (EndoVac Microcannula; Discus Dental, Culver City, CA)
Flexiglide needle for irrigation also easily follows curved canals
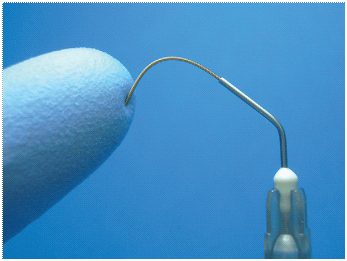
Plastic syringes of different sizes
Research articles on sonic irrigation, in chronological order [19-30]
| | Irrigation | Evaluation |
|---|
| Year | Author | Irrigation instrument | Irrigant | Evaluation method | Evaluation criteria |
|---|
| 1985 | Tronstad et al., | #20 K-file | 2.5% NaOCl | SEM | Smear layer, dentin debris |
| 1985 | Barnett et al., | #35 K-file | 15% EDTA | - | - |
| 1987 | Stamos et al., | #30 K-file | 2.6% NaOCl | Histologic evaluation | Pulpal tissue and dentin debris |
| 1987 | Reynolds et al., | #20 k file | Water | Histologic evaluation | Predentin and dentin debris |
| 1989 | Pugh et al., | - | Tap water | Injection with impression material and clearing | Canal morphology |
| 1989 | Walker and del Rio | #25, 20 Trisonic file #15 Endostar file | Tap water | Histologic evaluation | Debris |
| 2003 | Sabins et al., | #15 Rispisonic file | 5.25% NaOCl | Surgical operating microscope | Dentin debris |
| 2008 | Ruddle | EndoActivator tips | | | dentinal cleanliness |
Some of the important studies related to Endovac [15,16,37-51]
| Author | Year | Study | Results |
|---|
| John Schoeffel | 2007 | Intoduced EndoVac | New & efficient method of irrigation |
| Benjamin A. Nielsen et al., | 2007 | Endovac and conventional technique | Endovac showed better debridement |
| Brito et al., | 2009 | Enterococcus faecalis populations after endovac | No antibacterial superiority |
| Shin et al., | 2010 | Endovac with conventional irrigation needles | EndoVac left significantly less debris |
| Desai and Himel | 2009 | Endovac, Endoactvator and conventional method | Less extrusion in case of Endovac and Endoactvator |
| Cameron Townsend et al., | 2009 | Endovac, Endoactivator, sonic and conventional technique, | In a plastic simulated canal, ultrasonic agitation was significantly more effective than needle irrigation and Endo Vac irrigation at removing intracanal bacteria. |
| Susin et al., | 2010 | Canal and isthmus debridement efficacies of two irrigant agitation techniques | ANP removes more debris |
| Nestor. Cohenca, et al., | 2010 | Antibacterial efficacy of Endovac and conventional technique | Endovac showed promising results |
| De Gregorio et al., | 2010. | Efficacy of different irrigation and activation systems on the penetration of sodium hypochlorite | Endovac showed better penetration |
| Mitchell and Baumgartner | 2010 | Compared of apical extrusion of NaOCl | Less apical extrusion in endovac |
| Gondim et al.,. | 2010 | Postoperative pain after the application of two different irrigation devices | Less pain with Endovac |
| Miller et al., | 2010. | Antimicrobial efficacy of irrigation using the EndoVac superiority | No antibacterial |
| Cris siu et al., | 2010 | Endovac vs conventional | Better debridement with endovac at last 1 mm |
| Richard K Howard, et al., | 2011 | Endovac, I max probe,piezoflow | No significant difference in debris |
| Shehab El-Din Saber et al., | 2011 | endovac, passive ultrasonic irrigation (PUI) & manual dynamic agitation (MDA) | Endovac & MDA better in smear removal than PUI |
| Mohan Abarajithan et al., | 2011 | smear removal between endovac and conventional | Endovac showed better results |
| Hugo Roberto et al., | 2012 | PUI, Endovac and conventional | PUI and endovac are more effective than the conventional endodontic needle |
| Pawar et al., | 2012 | antimicrobial activity of endovac vs conventional | No significant difference |
| Ahmed Alkahtani et al., | 2014 | Endovac and conventional technique | Endovac produce less apical extrusion of Debris & more efficient in cleaning |
| Varsha et al., | 2013 | Endovac, ultrasonic irrigation & conventional technique | Apical extrusion of debris was least in Endovac |
| Fuat Ahmetoglu et al., | 2014 | Endovac, ultrasonic irrigation & conventional technique | EDTA should be used, regardless of the technique in each of the three |
Conclusion
Irrigation has a key role in successful endodontic treatment. Although NaOCl is the most important irrigating solution, no single irrigant can accomplish all the tasks required by irrigation. Technological advances like positive and negative irrigation have brought to fruition new devices that rely on various mechanisms of irrigant transfer, soft tissue debridement and, depending on treatment philosophy, removal of smear layers. Negative irrigation is although more superior to positive pressure irrigation as prevent periapical extrution of irrigant, provides better cleansing, has no vapor lock effect and provides adequate irrigant volume but still further research is warranted.
[1]. TW Chow, Mechanical effectiveness of root canal irrigationJ Endod 1983 9(1):475-79. [Google Scholar]
[2]. LS Gu, JR Kim, J Ling, A Choi, A Pashley, M Tay, Review of contemporary irrigant agitation techniques and devicesJ Endod 2009 35(6):791-804. [Google Scholar]
[3]. KW Falk, CM Sedgley, The influence of preparation size on the mechanical efficacy of root canal irrigation in vitroJ Endod 2005 31(10):742-45. [Google Scholar]
[4]. C Boutsioukis, C Gogos, B Verhaagen, M Versluis, E Kastri-nakis, LW Van der Sluis, The effect of root canal taper on the irrigant flow: evaluation using an unsteady Computational Fluid Dynamics modelInt Endod J. 2010 43(10):909-16. [Google Scholar]
[5]. C Boutsioukis, C Gogos, B Verhaagen, M Versluis, E Kastri-nakis, LW Van der Sluis, The effect of apical preparation size on irrigant flow in root canals evaluated using an unstea-dy Computational Fluid Dynamics model.Int Endod J 2010 43(10):874-81. [Google Scholar]
[6]. CM Sedgley, AC Nagel, D Hall, B Applegate, Influence of irrigant needle depth in removing bioluminescent bacteria inoculated into instrumented root canals using real-time imag-ing in vitroInt Endod J 2005 38(2):97-104. [Google Scholar]
[7]. C Boutsioukis, B Verhaagen, M Versluis, E Kastri-nakis, LW Van der Sluis, Evaluation of irrigant flow in the root canal using different needle types by an unsteady computa-tional fluid dynamics modelJ Endod 2010 36(5):875-9. [Google Scholar]
[8]. SM Al-Hadlaq, SA Al-Turaiki , Y Al-Sulami, AY Saad, Effi-cacy of new brushcovered irrigation needle in removing root canal debris: a scanning electron microscopic studyJ Endod. 2006 32(12):1181-84. [Google Scholar]
[9]. Haapasalo Markus, Irrigation in EndodonticsDent Clin N Am 2010 54:291-312. [Google Scholar]
[10]. NP Migun, MA Azuni, Filling of one-side-closed capillaries immersed in liquidsJ Colloid Interface Sci 1996 181:337-40. [Google Scholar]
[11]. F Bronnec, S Bouillaguet, P Machtou, Ex vivo assessment of irrigant penetration and renewal during the final irrigation regimenInt Endod J 2010 43(8):663-72. [Google Scholar]
[12]. TY Huang, K Gulabivala, YL Ng, A bio-molecular film ex-vivo model to evaluate the influence of canal dimensions and irri-gation variables on the efficacy of irrigationInt Endod J 2008 41(1):60-71. [Google Scholar]
[13]. ES Senia, FJ Marshall, S Rosen, The solvent action of sodium hypochlorite on pulp tissue of extracted teethOral Surg Oral Med Oral Pathol 1971 31:96-103. [Google Scholar]
[14]. M Abou-Rass, M Piccinino, The effectiveness of four clinical irrigation methods on the removal of root canal debrisOral Surg Oral Med Oral Pathol Oral Radiol Endod 1982 54(3):323-28. [Google Scholar]
[15]. Schoeffel G. John, The EndoVac Method of Endodontic Irrigation Part 2—Efficacy. Dentistry Today. January 2008 [Google Scholar]
[16]. Schoeffel G. John, The EndoVac Method of Endodontic Irrigation Part- 3: System Components and Their Interaction. Dentistry Today. July. 2008 [Google Scholar]
[17]. CJ Ruddle, Microbrush for endodontic useTetracyclineTeratology 2001 WashingtonDC: United States:617 [Google Scholar]
[18]. M Weise, MJ Roggendorf, J Ebert, A Petschelt, R Frankenberger, Four methods for cleaning simulated lateral extensions of curved root canals: a SEM evaluationInt Endod J 2007 40:991-92. [Google Scholar]
[19]. L Tronstad, F Barnett, L Schwartzben, P Frasca, Effectiveness and safety of a sonic vibratory endodontic instrumentEndod Dent Traumatol 1985 1:69-76. [Google Scholar]
[20]. M Ahmad, TR Pitt Ford, LA Crum, Ultrasonic debridement of root canals: an insight into the mechanisms involvedJ Endod 1987 13:93-101. [Google Scholar]
[21]. AD Walmsley, PJ Lumley, WR Laird, Oscillatory pattern of sonically powered endodontic filesInt Endod J 1989 22:125-32. [Google Scholar]
[22]. WG Pitt, Removal of oral biofilm by sonic phenomenaAm J Dent 2005 18:345-52. [Google Scholar]
[23]. F Barnett, R Godick, L Tronstad, Clinical suitability of a sonic vibratory endodontic instrumentEndod Dent Traumatol 1985 1:77-81. [Google Scholar]
[24]. DE Stamos, EM Sadeghi, GC Haasch, H Gerstein, An in vitro comparison study to quantitate the debridement ability of hand, sonic, and ultrasonic instrumentationJ Endod 1987 13:434-40. [Google Scholar]
[25]. MA Reynolds, S Madison, RE Walton, KV Krell, BR Rittman, An in vitro histological comparison of the step-back, sonic and ultrasonic instrumentation techniques in small, curved root canalsJ Endod 1987 13:307-14. [Google Scholar]
[26]. RJ Pugh, AC Goerig, CG Glaser, WJ Luciano, A comparison of four endodontic vibratory systemsGen Dent 1989 37:296-301. [Google Scholar]
[27]. TL Walker, CE del Rio, Histological evaluation of ultrasonic and sonic instrumentation of curved root canalsJ Endod 1989 15:49-59. [Google Scholar]
[28]. SA Jensen, TL Walker, JW Hutter, BK Nicoll, Comparison of the cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canalsJ Endod 1999 25:735-38. [Google Scholar]
[29]. RA Sabins, JD Johnson, JW Hellstein, A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canalsJ Endod 2003 29:674-78. [Google Scholar]
[30]. CJ Ruddle, Endodontic disinfection: tsunami irrigationEndod Practice 2008 :7-15. [Google Scholar]
[31]. TL Walker, CE del Rio, Histological evaluation of ultrasonic and sonic instrumentation of curved root canalsJ Endod 1989 15:49-59. [Google Scholar]
[32]. A Burleson, J Nusstein, A Reader, M Beck, The in vivo eval-uation of hand/ rotary/ultrasound instrumentation in necrotic, human mandibular molarsJ Endod 2007 33(7):782-87. [Google Scholar]
[33]. K Carver, J Nusstein, A Reader, M Beck, In vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molarsJ Endod 2007 33(9):1038-43. [Google Scholar]
[34]. A Lussi, U Nussba¨cher, J Grosrey, A novel noninstrumented technique for cleansing the root canal systemJ Endod 1993 19:549-53. [Google Scholar]
[35]. Y Fukumoto, I Kikuchi, T Yoshioka, C Kobayashi, H Suda, An ex vivo evaluation of a new root canal irrigation technique with intracanal aspirationInt Endod J 2006 39:93-99. [Google Scholar]
[36]. S McGill, K Gulabivala, N Mordan, YL Ng, The efficacy of dynamic irrigation using a commercially available system (Rin-sEndo) determined by removal of a collagen 'bio-molecular film' from an ex vivo modelInt Endod J 2008 41(7):602-08. [Google Scholar]
[37]. BA Nielsen, J Craig Baumgartner, Comparison of the Endo-Vac system to needle irrigation of root canalsJ Endod 2007 33(5):611-15. [Google Scholar]
[38]. JL Hockett, JK Dommisch, JD Johnson, N Cohenca, Antimi-crobial efficacy of two irrigation techniques in tapered and nonta-pered canal preparations: an in vitro studyJ Endod 2008 34(11):1374-77. [Google Scholar]
[39]. SJ Shin, HK Kim, IY Jung, Comparison of the cleaning efficacy of a new apical negative pressure irrigating system with conventional irrigation needles in the root canalsOral Surg Oral Med Oral Pathol Oral Radiol Endod 2010 109(3):479-84. [Google Scholar]
[40]. C Townsend, J Maki, An In Vitro Comparison of New Irrigation and Agitation Techniques to Ultrasonic Agitation in Removing Bacteria From a Simulated Root CanalJ Endod. 2009 35:1040-43. [Google Scholar]
[41]. L Susin, Y Liu, JC Yoon, Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed systemInt Endod J 2010 43(12):1077-90. [Google Scholar]
[42]. PR Brito, LC Souza, JC Machado de Oliveira, Comparison of the effectiveness of three irrigation techniques in reducing intracanal Enterococcus faecalis populations: an in vitro study J Endod 2009 35(10):1422-27. [Google Scholar]
[43]. C De Gregorio, R Estevez, R Cisneros, Efficacy of different irrigation and activation systems on the penetration of sodium hypochlorite into simulated lateral canals and up to working length: an in vitro studyJ Endod 2010 36(7):1216-21. [Google Scholar]
[44]. RP Mitchell, SE Yang, JC Baumgartner, Comparison of apical extrusion of NaOCl using the EndoVac or needle irrigation of root canalsJ Endod 2010 36(2):338-41. [Google Scholar]
[45]. E Gondim, F Setzer, C Bertelli dos Carmo, Postoperative pain after the application of two different irrigation devices in a prospective randomized clinical trialJ Endod 2010 36(8):1295-301. [Google Scholar]
[46]. TA Miller, JC Baumgartner, Comparison of the antimicrobial efficacy of irrigation using the EndoVac to endodontic needle deliveryJ Endod 2010 36(3):509-11. [Google Scholar]
[47]. rekha Pawar, Abdullah Alqaied, Safavi Kamran, Influence of an Apical Negative Pressure Irrigation Systemvon Bacterial Elimination during Endodontic Therapy: A Prospective Randomized Clinical StudyJ Endod 2012 38:1177-81. [Google Scholar]
[48]. Tambe Varsha H, Nagmode Pradnya S, Vishwas Jayshree R, Evaluation of the Amount of Debris extruded apically by using Conv-entional Syringe, Endovac and Ultrasonic Irrigation Technique: An In Vitro StudyJournal of International Oral Health 2013 5(3):63-66. [Google Scholar]
[49]. Desai Pranav, Himel Van, Comparative Safety of Various Intracanal Irrigation SystemsJ Endod 2009 35:545-49. [Google Scholar]
[50]. Ahmetoglu Fuat, Keles Ali, Yalcin Muhammet, Simsek Neslihan, Effectiveness of different irrigation systems on smear layer removal: A scanning electron microscopic studyEuropean Journal of Dentistry 2014 8(1):53-57. [Google Scholar]
[51]. Schoeffel G. John , The EndoVac Method of Endodontic Irrigation – safety first. Dentistry Today. october 2007 [Google Scholar]