Evaluation of Potential Drug - Drug Interactions in General Medicine Ward of Teaching Hospital in Southern India
Akram Ahmad1, Muhammad Umair Khan2, Irfanul Haque3, Rahul Ivan4, Ram Dasari5, Megha Revanker6, A. Pravina7, Sheetal Kuriakose8
1 Lecturer, Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, UCSI University, Cheras, Kuala Lumpur, Malaysia.
2 Lecturer, Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, UCSI University, Cheras, Kuala Lumpur, Malaysia.
3 Pharm D Intern Students, Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore, Karnataka, India.
4 Pharm D Intern Students, Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore, Karnataka, India.
5 Pharm D Intern Students, Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore, Karnataka, India.
6 Pharm D Intern Students, Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore, Karnataka, India.
7 Pharm D Intern Students, Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore, Karnataka, India.
8 Assistant Professor, Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Irfanul Haque, Pharm D Intern Department of Pharmacy Practice, The Oxford College of Pharmacy, Bangalore-560068, Karnataka, India. E-mail : Irfaanpharma@gmail.com
Background: Polypharmacy is considered as one of the major risk factors in precipitation of drug-drug interactions (DDIs). Patient population at high risk include the elderly and patients with co morbidities as they are usually prescribed with more number of drugs. Critical evaluation of such prescriptions by pharmacist could result in identification and reduction of such problems.
Objective: The study aims to assess the prevalence, severity and significance of potential DDI (pDDI) in general medicine wards of South Indian tertiary care teaching hospital.
Materials and Method: A prospective observational study was conducted in a general medicine ward for a period of six months (September 2012 to February 2013). The socio-demographic, clinical characteristics and medication prescribed was documented in a specially designed form. Analysis was carried out to assess the prevalence, severity and significance of identified pDDIs using Micromedex. Descriptive and Univariate analysis were used to report the findings.
Results: A total of 404 case records reviewed, 78 (19.3%) patients had pDDIs. A total of 139 (34.4%) pDDIs were reported during the study period. Majority (53.95%, n=75) of the interactions were moderate in intensity and significant in nature (53.23%, n=74). Positive association between number of pDDIs and age was observed.
Conclusion: The prevalence of pDDIs was 19.3% which is lesser then previously reported studies from India. Patient with more co-morbidities and elders were observed with more pDDIs. The study highlighted the need to effectively monitor and patients prevent pDDIs to improve patient safety.
Drug-drug interaction, Hospitalised patients, Polypharmacy
Introduction
Drug therapy becomes more complex with polypharmacy. Such prescriptions need to be evaluated thoroughly in order to avoid any chances of drug related problems (DRPs). DRPs lead to increase morbidity, mortality and increase healthcare expenses [1,2]. The involvement of pharmacist in a health care system gives the opportunity to cater these demands and it gives the opportunity to involve greatly in the provision of drug related therapy which is not only effective but also free from any kind of toxicity. Drug-Drug interaction (DDI) is one of the kinds of drug related problems in which effects of one drug can be altered by the co-administration of another drug [3]. Drug-drug interactions can be classified from different perspectives as it mechanistically gives an insight of how to predict, detect and avoid them. Pharmacokinetic and Pharmacodynamic interactions are important types of drug interactions classified on the basis of mechanism of action in clinical practice. In pharmacokinetic interactions, one drug affects the absorption, distribution, metabolism and excretion of other drug. While in pharmacodynamics interaction, two or more drugs may have additive or antagonistic effects [4].
DDIs are termed as pharmacological and clinical outcomes resulted from simultaneous use of different combinations of drug as compared to their use alone. These DDIs could result in serious life threatening conditions in a desire to alter the therapeutic end point of drugs. DDI monitoring not only applicable on those drugs which are contraindicated, but the required monitoring and the adjustment of dose should also be deemed essential for those combinations which are considered beneficial for certain conditions. Therefore, it is essential to identify possible DDIs in clinical settings and approach towards the management of potential loss of effectiveness and appearance of toxicity because of the use of certain drug combinations [5].
Clinical pharmacist occupies an important position in healthcare settings as it gets an opportunity to work in a team and utilize the professional skills, knowledge and expertise for better patient care. Among the various professional services provided by the pharmacists, monitoring DRPs like pDDIs is the most important one as it helps in improving patient safety in hospital settings. Since DDIs are an important cause for increase in morbidity and mortality rates in hospitalised patients [6], it is imperative to assess the insight of pDDIs in hospitalised patients [7].
The frequency with which therapeutic and other types of incompatibilities taking place can be drastically reduced if multiple drug therapy is always prescribed rationally and only when essential. The hazards resulting from the large number of drugs received over relatively short periods of time by many patients have been well documented [6]. All the members of the health care team need to be alert to prevent therapeutically incompatible medications from reaching patients [8]. It is utmost need not only to maintain complete and current patient medication records, but also to supervise and monitor drug therapy more closely by placing the pharmacist in clinical settings to detect and prevent DDIs [7,8].
The issue of drug interactions is a global concern. A study from US reported that 30.3% patients were at risk of DDIs in ambulatory care unit [9]. Other studies have also reported high rate prevalence of drug interactions worldwide [10–15]. In India, a study identified 66% of DDIs in a medicinal department of a tertiary care hospital in Karnataka, India [16]. While, another study in Chandigarh reported that 8.3% prescriptions had multiple DDIs [17]. In view of above mentioned statistics, we purposefully conducted this study in the private sector hospital of Bangalore as not many studies targeted the private sector in this region of the country. The aim of the study is to analyse the prevalence and nature of pDDIs observed in hospitalised patients from the general medicine ward of the study hospital.
Materials and Methods
Study site, design and duration
The study was conducted in the general medicine ward of 1000 bed teaching referral hospital providing primary and specialized health care for people in and around Bangalore district. This was a prospective observational study which was conducted for the period of 6 months (September 2012 to February 2013). In a specially designed form all clinical and demographic information were collected from patients’ case notes, treatment charts, interviewing patients or caretakers, interviewing health care professionals and other relevant sources. An inform consent form was given to the patients. The study was also approved by principal of The Oxford College of pharmacy, Bangalore, India.
Patient of both gender aged more than 18 years old, who admitted and received more than 24 hours inpatient services with two or more medications were included in this study. Patients with less than 24 hours inpatient services and patient with medical disability were excluded. The sample size of 377 was calculated on the basis of Raosoft [18] software in which the power was kept as 80%, response distribution as 50%, while confidence interval and margin of error was set at 95% and 5% respectively. However, during study period we were able to review 404 profiles which were well above the minimum requirement of a sample size.
Criteria for level of significance: Each pDDI was categorized according to their level of significance (LOS). LOS relates to the magnitude of the effect, to the likelihood of occurrence, and subsequently, to the necessity of monitoring the patient or altering therapy to avoid potentially adverse consequences. The LOS for the pDDIs was determined from the Micromedex software as analysed in previous study [19].
Criteria for severity [19]: It is an essential component of DDI study to assess and categorize interactions on the basis of severity. It enhances the decision making ability by assessing the risk vs benefit alternatives. However, by slight modification in posology of drugs, these potential interactions could be prevented. On the basis of severity, drug-drug interactions are categorized in minor, moderate and severe.
Minor drug interactions do not result in any significant troublesome outcomes. Management of these types of interactions is usually not required. Moderate drug interactions could result worsening in clinical condition of patient. Treatment to manage such type of interactions could be considered. Major drug interactions could lead to life threatening condition, therefore it should be considered essential to counter such problems as soon as they are identified.
Estimation of the Frequency of pDDIs: The prevalence of pDDIs was estimated by using the following formula:
Frequency of DDIs = Total no. of pDDIs/ Total no. of patients x 100 [20]
Results
A total of 404 patient’s case records were reviewed in general medicine ward during six months study period in which 214 (53%) patients were males and 190 (47%) patients were females. The mean age of patients was (48±17.93) ranging from 18 to 95 y. Out of 404 case records reviewed, 139 (34.4%) DDIs were identified and 78 (19.3%) patients had pDDIs. The number of drugs was ranging from 3 to 10 drugs (Mean+ SD: 6±2.13). Majority (54%) of the patients presented enrolled in this had some kind of past medical problems. The demographic characteristics of patients with DDIs are presented in [Table/Fig-1].
Demographic characteristics of patients with pDDIs
| Characteristics | n (%) |
|---|
| Total pDDIs | 78 |
| Age in years | |
| 18-40 | 26 (33.3) |
| 40-60 | 30 (38.4) |
| >60 | 22 (28.2) |
| Gender | |
| Males | 37 (47.4) |
| Females | 41 (52.5) |
| No. of drugs | |
| 2- 4 | 12 (15.3) |
| 5-7 | 36 (46.1) |
| 8-10 | 30 (38.4) |
| Prior medical problems | |
| Patients with prior medical Problems | 220 (54) |
| Patients without prior medical problems | 184 (46) |
The study shows that most frequent drug interaction was between paracetamol and pantoprazole 25 (17.98%), followed by ofloxacin-ondansetron 21 (15.1%) theophylline-budesonide 19 (13.66%), ibuprofen-ofloxacin 9 (6.47%), Mefenamic acid-atenolol/amlodipine 12 (8.63%), frusemide-aspirin 9(6.4%). The complete description has been given [Table/Fig-2].
Classification of pDDIs according to Micromedex software
| Interacting Drugs | Total no (%age) | ASIA grade A | No. of DDIs n=139(%) |
|---|
| Paracetamol- Pantoprazole | Rapid | Moderate | 25 (17.98) |
| Frusemide- Aspirin | Delayed | Moderate | 9 (6.47) |
| Ofloxacin- Ondansetron | Rapid | Major | 21 (15.1) |
| Theophylline- Budesonide | Delayed | Major | 19 (13.66) |
| Aspirin-Ramipril/Lisinopril/Captopril | Rapid | Moderate | 7 (5.03) |
| Paracetamol- Phenytoin | Delayed | Moderate | 5 (3.59) |
| Iron- Omeprazole/Pantoprazole | Rapid | Moderate | 9 (6.47) |
| Antacid-Digoxin | Rapid | Moderate | 9 (6.47) |
| Ibuprofen- Furosemide | Delayed | Minor | 8 (5.75) |
| Levofloxacin- Insulin | Rapid or delayed | Major | 4 (2.87) |
| Frusemide- Digoxin | Rapid | Moderate | 2 (1.43) |
| Ibuprofen- Ofloxacin | Delayed | Moderate | 9 (6.47) |
| Mefenamic acid- Atenolol/Amlodipine | Delayed | Minor | 12 (8.63) |
The study also highlighted on the severity of pDDIs and found that majority of 75 (53.95%) pDDIs were moderate in nature, 44 (31.65%) were found to be severe and 20 (14.38%) were found to be mild [Table/Fig-3].
Data showing severity of drug interactions
| Severity | Number n=139 (%) |
| Minor | 20 (14.38) |
| Moderate | 75 (53.95) |
| Major | 44 (31.65) |
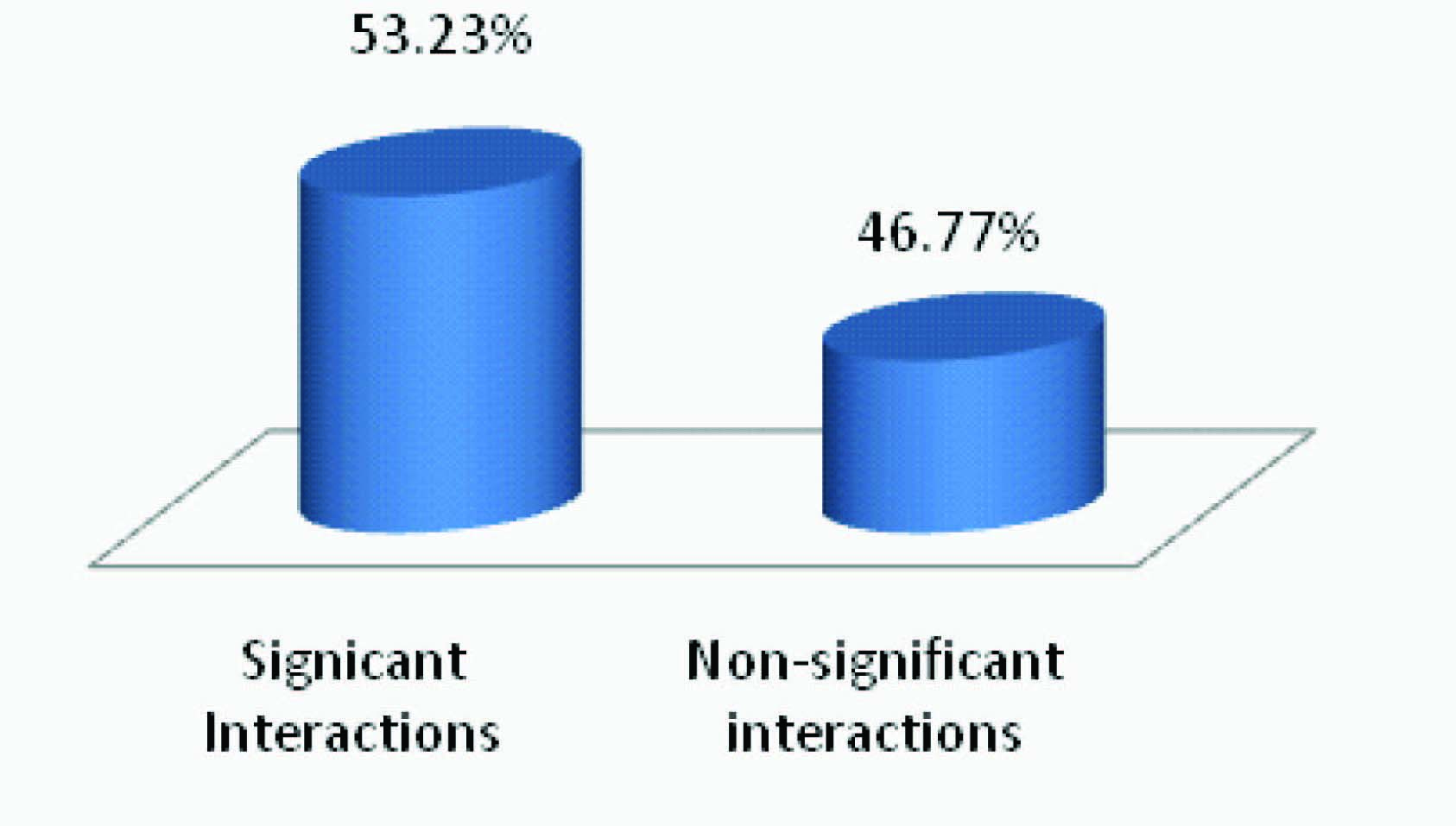
The study revealed that more than half of the pDDIs were significant in nature as depicted in [Table/Fig-4]. The findings of this study revealed that the prevalence of pDDIs were higher in pain/fever prescriptions (25.1%). Conversely, least number of pDDIs was observed in arthritis patients (2.1%). While the prevalence of pDDIs in prescriptions with respiratory disease, cardiovascular disease, GI problems, Diabetic issues and skin reactions were 17.9%, 15.8%, 12.9%, 15.1% and 3.5% respectively [Table/Fig-5].
Level of significance of pDDIs
Number of pDDIs prescription in each disease
| Disease | Total No. of prescriptions | Total No. of drug interactions (%) |
|---|
| Fever/ Pain | 86 | 35 (25.1) |
| Respiratory System | 66 | 25 (17.9) |
| Cardiovascular System | 72 | 22 (15.8) |
| GIT system | 44 | 18 (12.9) |
| Diabetes mellitus | 62 | 21 (15.1) |
| Arthritis | 14 | 3 (2.1) |
| Skin reactions | 18 | 5 (3.5) |
| Tuberculosis | 12 | 5 (3.5) |
| Central nervous system | 30 | 5 (3.5) |
Discussion
In the present study we observed a range of pDDIs among adult inpatients in medicine ward of study hospital. The prevalence of pDDIs in medicine department is found to be 19.5% which is lesser than those reported previously [20,21]. This study found that higher rate of pDDI was found in women between 40-60 years of age. These results are in line with other studies which also showed that pDDIs are more frequent in older patients who are on polypharmacy [22–24]. The possible reason could be due to the reason of higher comorbidities in older patient which increases the number of drugs in prescription and thus the chances of pDDIs also increases. This argument is supported by the same results reported by Snaith et al., [25], in an observation study which revealed the optimistic association between the number of drugs prescribed and length of stay with DDIs [1,21].
In this study, we found that most common drug classes involved in DDIs were NSAIDs, Antibiotics, Proton Pump Inhibitors, Corticosteroids etc. Among these drug classes, paracetamol and pantaprozole 27(19.4%), ofloxacin and ondansetron 24 (17.2%), theophylline and budesonide 22 (15.8%), ibuprofen and ofloxacin 11 (7.9%) and mafenamic acid and atenolol/amlodipine 12(8.6%) were the most commonly observed drug pairs resulting in DDIs. Similar results were observed in an another study conducted by shah et al.,[19].
The results suggests that majority of DDIs were associated with fever/pain prescription followed by respiratory and cardiovascular system. The results are in line with other previous studies which have reported high incidence of DDIs in above mentioned areas. A study reported 30.67% pDDIs in cardiology ward of a tertiary hospital [26]. World Health Organization (WHO) reported that number of cardiology patients will rise to about 69 million cases in the year 2015. With this escalating increase in number of patients, it is essential to design some essential interventions in order to address this issue [27]. Similarly, fever and pain drugs are most frequently prescribed drugs in a hospital that makes them at risk of DDIs. The results of this study also highlight this area as most of the DDIs were identified in such prescriptions. Awareness program must be raised by stakeholders to educate health professionals regarding the rational use of drugs in pain/fever conditions. This statement is also supported by Hersh et al., in their attempt to explore this issue [28]. Likewise, prevalence of DDIs was also high in respiratory prescriptions. Not many researchers have explored this area; however, there is a need to conduct future research on this topic as it can be speculated that DDIs could be a troublesome feature of respiratory prescriptions [29].
With everyday passing by a new drug is coming in market and the availability of multiple options can drag prescriber towards polypharmacy which increases the chances of pDDIs. There is a need to raise the awareness of possible DDIs in all hospital departments and all pDDI should be identified, managed and recorded. The age, gender, polypharmacy and co-morbidities are known as the risk factors for developing DDIs. These findings were similar to previously reported studies [5,19,21].
Furthermore, it has been observed that use of polypharmacy was related to widely increased risk of unsafe drug-drug combinations [30,31]. Management of DDIs, medication withdrawal, change with another alternative or dose reduction should be the first step to be employed for the rectification of this problem. Developing methods like check list/trigger tools would be more helpful to identify and prevent drug related problems. These methods are cost effective and improve the patient safety in hospital setups [32,33].
The results of this study brought into light an important aspect for future research which is focussed on geriatric population. This study has revealed that this sect of group is more prone to drug interactions, thus it is the need of time to explore this area in order to promote safe, and effective therapies without any drug related problems like drug-drug interactions.
Limitation
Limitation of this study is its short duration without any intervention component. Controlled study to evaluate whether good clinical management of DDIs can reduce drug-related morbidity or mortality is needed in the future in this discipline.
Conclusion
The study was conducted to assess the DDIs in the hospitalized patients of general medicine wards of tertiary care hospital. The result of the study showed frequency of DDIs to be 19.3%. The study concluded that DDIs are more prevalent in patients suffering from co-morbidities due to increase number of drugs in their prescription. The frequency of drug interactions could have been less with a more judicial use of the drugs. This study has highlighted the need for future studies to be conducted in order to improve the prescribers’ awareness on DDIs and their management in improving the clinical outcome.
[1]. Rajakannan T, Mallayasamy S, Guddattu V, Kamath A, Vilakkthala R, Rao PG, Cost of adverse drug reactions in a South Indian tertiary care teaching hospitalJ Clin Pharmacol 2012 52(4):559-65. [Google Scholar]
[2]. Akram Ahmad, Parimalakrishnan S, Mohanta GP, Manna PK, Manavalan R, Incidence of Adverse Drug Reactions With Commonly Prescribed Drugs in Tertiary Care Teaching Hospital in IndiaInt J Res Pharm Sci 2012 3(1):1-5. [Google Scholar]
[3]. Tipnis HP, Amrita B, Text book of Clinical Pharmacy20031st ediCareer Publication:274-75. [Google Scholar]
[4]. Mounica B, A Prospective Study on Drug-Drug Interactions In The Medication Charts in General Medicine wards, in a Tertiary Care Hospital, Guntur, Andhra Pradesh and The Clinical Pharmacists RoleInt J Biolog Pharm Res 2014 5(4):374-77. [Google Scholar]
[5]. Arvind K, Churi Shobha, Assessment of drug-drug interactions in hospitalizes patients in IndiaAsian J Pharm Clinical Res 2011 1(4):62-65. [Google Scholar]
[6]. Nekkanti H, Mateti UV, Vilakkathala R, Rajakannan T, Mallayasamy S, Padmakumar R, Predictors of warfarin-induced bleeding in a South Indian cardiology unitPerspect Clin Res 2012 3(1):22-25. [Google Scholar]
[7]. Ankur R, Ashutosh P, Singhraj Y, Drug interaction: A Succinct ReviewInt J Pharm Chem Sci 2013 1(2):297-302. [Google Scholar]
[8]. Bertoli R, Bissig M, Caronzolo D, Assessment of potential drug drug interactions at hospital dischargeSwiss Med Wkly 2010 140:w13043 [Google Scholar]
[9]. Delafuente JC, Understanding and preventing drug interactions in elderly patientsCrit Rev Oncol Hematol 2003 48(2):133-43. [Google Scholar]
[10]. Costa AJ, Potential drug interactions in an ambulatory geriatric populationFam Pract 1991 8(3):234-36. [Google Scholar]
[11]. Ratz Bravo AE, Tchambaz L, Krahenbuhl-Melcher A, Hess L, Schlienger RG, Krahenbuhl S, Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapyDrug Saf 2005 28(3):263-75. [Google Scholar]
[12]. Gosney M, Tallis R, Prescription of contraindicated and interacting drugs in elderly patients admitted to hospitalLancet 1984 2(8402):564-67. [Google Scholar]
[13]. Manchon ND, Bercoff E, Lemarchand P, Chassagne P, Senant J, Bourreille J, Incidence and severity of drug interactions in the elderly: a prospective study of 639 patientsRev Med Interne 1989 10(6):521-25. [Google Scholar]
[14]. Wiltink EH, Medication control in hospitals: a practical approach to the problem of drug-drug interactionsPharm World Sci 1998 20(4):173-77. [Google Scholar]
[15]. Gronroos PE, Irjala KM, Huupponen RK, Scheinin H, Forsstrom J, Forsstrom JJ, A medication database-a tool for detecting drug interactions in hospitalEur J Clin Pharmacol 1997 53(1):13-17. [Google Scholar]
[16]. Shahabudin S, Bharti C, Faizal P, Surveillance of The Potencial Drug-Drug Interactions in The Medicine Department of a Tertiary Care HospitalJ Clin Diag Research 2012 6(7):1258-61. [Google Scholar]
[17]. Kashyap Mandavi, D’Cruz Sanjay, Sachdev Atul, Tiwari Pramil, Drug-drug interactions and their predictors: Results from Indian elderly inpatientsPharm Pract (Granada) 2013 11(4):191-95. [Google Scholar]
[18]. Raosoft. An Online Sample Size Calculator; 2008. Available from http://www.raosoft.com/samplesize.html [Accessed on 10/11/2014] [Google Scholar]
[19]. Shah S, Naqvi BS, Zehra A, Ali D, Saeed R, Naqvi GR, Quantitative Analysis of Drug- Drug Interactions of OTC Drugs with other Prescribed Drugs Collected from Different Hospitals and Clinics of Karachi, PakistanJ Pharm Sci 2011 4(2):137-48. [Google Scholar]
[20]. Adepu Ramesh, Singhal Rohit, Nagavi B.G, A Study of Potential Drug-Drug Interactions in Prescriptions Received at Selected Community PharmaciesIndian J Pharm Educ Res 2008 42(2):184-89. [Google Scholar]
[21]. Mateti UV, Rajakannan T, Nekkanti H, Rajesh V, Mallaysamy SR, Ramachandran P, Drug-drug interactions in hospitalized cardiac patientsJ Young Pharmacists 2011 3:329-33. [Google Scholar]
[22]. Kaur S, Kapoor V, Mahajan R, Lal M, Gupta S, Monitoring of incidence, severity, and causality of adverse drug reactions in hospitalized patients with cardiovascular diseaseIndian J Pharmacol 2011 43(1):22-26. [Google Scholar]
[23]. Bjorkman IK, Fastbom J, Schmidt IK, Bernsten CB, Drug-drug interactions in the elderlyAnn Pharmacother 2002 36:1675-81. [Google Scholar]
[24]. Kohler G, Bode-Boger S, Busse R, Hoopmann M, Welte T, Boger R, Drug-drug interactions in medical patients: Effect of in-hospital treatment and relation to multiple drug useInt J ClinPharmacol Ther 2000 38:504-13. [Google Scholar]
[25]. Snaith A, Pugh L, Simpson CR, McLay JS, The potential for interaction between warfarin and co-prescribed medication: A retrospective study in primary careAm J Cardiovasc Drugs 2008 8:207-12. [Google Scholar]
[26]. Patel VK, Acharya LD, Rajakannan T, Surulivelrajan M, Guddattu V, Padmakumar R, Potential drug interactions in patients admitted to cardiology wards of a south Indian teaching hospitalAustralas Med J 2011 4(1):9-14. [Google Scholar]
[27]. Disease Burden in India. Estimations and causal analysis. Available at: http://www.whoindia.org/LinkFiles/Commision_on_Macroeconomic_and_Health_Bg_P2Burden_of_Disease_Estimations_and_Casual_analysis.pdf. [Accessed on 10/11/2014]. Frazier SC [Google Scholar]
[28]. Hersh EV, Pinto A, Moore PA, Adverse drug interactions involving common prescription and over-the-counter analgesic agentsClinical therapeutics 2007 29(11):2477-97. [Google Scholar]
[29]. Tobia CC, Aspinall SL, Good CB, Fine MJ, Hanlon JT, Appropriateness of antibiotic prescribing in veterans with community-acquired pneumonia, sinusitis, or acute exacerbations of chronic bronchitis: a cross-sectional studyClinical therapeutics 2008 30(6):1135-44. [Google Scholar]
[30]. Malone DC, Hutchins DS, Haupert H, Hansten P, Duncan B, Van Bergen RC, Assessment of potential drug-drug interactions with a prescription claims databaseAm J Heath Syst Pharm 2005 62:1983-91. [Google Scholar]
[31]. Jose J, Rao P, Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospitalPharma Res 2006 54:226-33. [Google Scholar]
[32]. Thiyagu R, Mallayasamy SR, Rajesh V, Varma Muralidhar, Prabhu Smitha, Vidyasagar Sudha, Development of indicators for identifying adverse drug events in an Indian tertiary care teaching hospitalDrug Healthc Patient Saf 2010 2:95-100. [Google Scholar]
[33]. Rajesh V, Surulivelrajan M, Vijayanarayana K, Sravanthi L, Pranitha M, Rodrigues G, Development of Trigger Tool for Identifying Adverse Events in Surgery: Experience of a Pilot StudyAsian J Pharm Health Sci 2013 3(3):791-99. [Google Scholar]