Background: Impaired balance between cell proliferation and apoptosis is crucial to the development of malignant neoplasm. The purpose of this study was to evaluate and compare the expression of X-Linked inhibitor of apoptotic protein (XIAP) (antiapoptotic marker) and Ki-67 (proliferative marker) expression in benign and malignant salivary gland (SG) tumours.
Materials and Methods: The study consisted of 40 cases of benign SG tumours and 50 cases of malignant SG tumours. The immunohistochemistry was carried out by using Ki-67 antibody (clone MIB-1) and XIAP antibody in all the groups.
Results: XIAP expression was significantly higher in malignant SG tumours than benign SG tumours (p = 0.016). Ki-67 LI was significantly higher in malignant SG tumours than benign SG tumours (p = 0.0002). Statistically significant positive correlation between Ki-67 count and XIAP expression was noted in benign and malignant SG tumours (p = 0.000).
Conclusion: As the expression of an antiapoptotic marker (XIAP) increases, the expression of a proliferative marker (Ki-67) also increases from benign to malignant SG tumours. Thus, targeted therapy of XIAP may play a future role in the management of SG malignancy.
Introduction
SG tumours, which represent 1% to 4% of all human neoplasias, affect the parotid gland in more than 70% of cases, with the submandibular gland (5% - 10%), the sublingual gland (1%), and the minor glands (5%-15%) sharing the rest [1]. These tumours have variable histopathologic and biologic characteristics [2].
Tumorogenesis involves a loss of balance between regulators of cell proliferation and apoptosis [3]. Apoptotic cell death plays an important physiological role for normal development and tissue homeostasis. Dysregulation of apoptosis has been implicated in carcinogenesis, tumour progression and resistance of tumour cells to chemotherapy [4].
Among the regulators of apoptosis, an evolutionary conserved gene family of inhibitor of apoptotic protein (IAP) has been identified and implicated in caspase inhibition. In humans, four IAPs (XIAP, c-IAP1, c-IAP2, and survivin) have been shown to restrain cell death in cancer cells through a mechanism initially thought to involve only inhibition of the effectors caspase-3 and -7 [5].
XIAP, considered to be the most potent IAP, inhibits caspases 3, 7, and 9, thereby blocking both the intrinsic (mitochondria-mediated) and extrinsic (death receptor– mediated) apoptotic pathways [6,7].
Till date, numerous studies has been reported which states the expression of number of biological markers,’ such as P53, HER2/c-erbB-2, BCL-2, Ki-67, p63 in numerous SG tumours [8–13] and only XIAP was used to delineate the process of malignant transformation of Pleomorphic adenoma to Carcinoma ex-pleomorphic adenoma (CXPA) [14].
Clinically, increased XIAP has been correlated with decreased survival in diffuse large B-cell lymphoma, adult and childhood acute myelogenous leukaemia, and renal cell carcinoma. [4,15–18]. Thus, the biological activity and central role in the caspase cascade makes the control of XIAP expression a promising molecular target for oncological therapy [14].
Ki-67 is the most commonly used cell proliferation markers. This antigen is present in all the active parts of cell cycle - G1, S, G2, M phase and absent in G0 phase. Its expression increases with cell cycle progression and reaches its peak during the G2 and M phases [19].
In this study, we evaluated the expression of XIAP, an antiapoptotic marker and its correlation with Ki-67 expression, a proliferative marker in benign and malignant SG tumours and also a possible future role for targeted therapy of XIAP in the management of these tumours.
Materials and Methods
The present study was carried out at the Department of Oral and Maxillofacial Pathology and Microbiology. The study protocol was approved by our Institutional Ethical Committee. The tumours studied were preselected based on the diagnosis and from the biopsy material acquired from last two years from both major and minor SG. The tumours included 40 cases of benign and 50 cases of malignant SG tumours [Table/Fig-1]. The 40 cases of benign tumours consisted of 20 pleomorphic adenoma, 05 monomorphic adenoma, 08 Warthin’s tumours and 07 canalicular adenoma. The 50 cases of malignant tumours consisted of 26 mucoepidermoid carcinoma of which 11 were high grade type, 08 were low grade type and 06 were of intermediate type, 14 adenoid cystic carcinoma and 06 Acinic cell carcinoma, 02 CXPA and 02 Polymorphous low grade carcinoma. Immunohistochemistry was performed on tissues fixed in 10% neutral buffered formalin, paraffin embedded tissue. The sections were cut serially to 5 μm for immunohistochemistry to evaluate expression of XIAP and Ki-67 antigens.
Expression of XIAP and Ki-67 count in the Study Groups
| Groups | Total No. | Total Positive (Ki-67) | Total Positive (XIAP) |
|---|
| Benign | 40 | 16 (40%) | 04 (10%) |
| Malignant | 50 | 24 (48%) | 13 (26%) |
Immunohistochemical Method for Detection of XIAP and Ki-67 Antigen
For immunohistochemistry, Peroxidase Detection System (Streptavidin-Biotin Detection System HRP-DAB; Product Code: RE7110K, Novo- castra kit) was employed. Endogenous peroxidase activity was blocked by treating hydrated sections with 3% H2O2 in methanol for 30 min. The slides were heated in a microwave oven for 10 min in 0.01M sodium citrate buffer (pH 6.0) for antigen retrieval and bench cooled for 20 min and again the same cycle was repeated. To prevent non-specific reactions, sections were incubated with 10% serum for 10 min. Pre-diluted Ki-67 antibody (clone MIB-1; Product code: N1633, Dako, Denmark) and diluted at 1:50 XIAP antibody (Cell Signaling technology, USA, cat # 2042) were incubated at room temperature in a humidifying chamber for 60 min and then at 4°C overnight. CXPA is generally an aggressive high-grade carcinoma arising in association with a primary or recurrent PA. Thus, known histopathological sections of CXPA revealed good expression of XIAP and Ki-67 antigen which was used as a positive control. One section from each positive control was used during each batch of immunohistochemistry procedure. This was followed by incubation with secondary biotinylated antibody and streptavidin-peroxidase reagent at room temperature in a humidifying chamber for 30 min. Freshly prepared substrate/chromogen solution of 3, 3’ Diaminobenzidine (mixing 5 ml of concentrated DAB in 50 ml of substrate buffer) was used to visualize the antigen–antibody reaction. Finally, the sections were counterstained in Mayer’s hematoxylin.
Assessment of Immunohistochemically Stained Sections
Assessment of immunohistochemical stained sections were performed by 05 trained histopathologist and average was taken into consideration.
Ki-67 – The cells were considered positive for Ki-67 antigen if there was an intra-nuclear DAB staining (brown colour). All the stained nuclei were scored positive regardless of their intensity of staining. Cells that lacked a clear nucleus were excluded. Minimum of 1000 cells were counted in each section. Tissue sections were scanned at 100 magnification for most heavily labelled Ki-67. The cell counts were made on captured image at 400 magnification with conventional light microscope in 10 randomly selected fields. Ki-67 labelling index (Ki-67 LI) was calculated from the ratio of the number of tumour cells stained by Ki-67 to the total number of tumour cells counted per section.
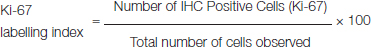
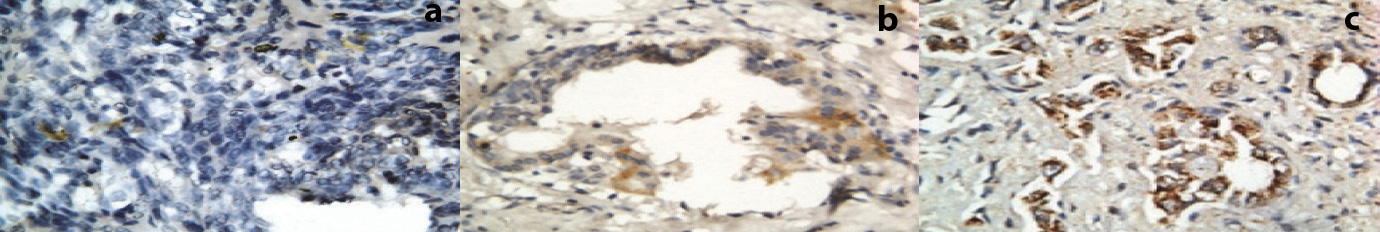
XIAP – The cells were considered positive for XIAP antigen if there was granular cytoplasmic staining (brown colour) as in agreement to Benjamin et al., [14]. Tissue sections were scanned at 100 magnification for most heavily labelled XIAP. Granular cytoplasmic staining was considered positive and intensity was graded at 400 magnification which were made on captured image with conventional light microscope in four to five fields randomly selected. The intensity was graded as 0 = no positive cells, 1 = 1–33% positive cells (mild), 2 = 34–66% positive cells (moderate), and 3 = 67–100% positive cells (strong).
Statistical Aanalysis
Group means for XIAP and Ki-67 LI were derived for each group. The data was analysed statistically using SPSS 16.0 version software for Windows, Mann-Whitney U-test and Pearson’s rank correlation analysis test. The level of statistical significance is at p < 0.05.
Results
Ki-67 LI
Ki-67 LI in benign SG tumours found to be ranging from 0.00 to 1.70 and in malignant SG tumours was 7.60 (±5.40). Ki-67 antigen was expressed in 40% of benign and 48% of malignant SG tumours. It was expressed in both epithelial cells as well as myoepithelial cells.
Descriptive statistics for Ki-67 expression between Benign and malignant SG tumours was done. Statistically significant difference was seen with p-value (p = 0.000) for KI-67 expression between benign and malignant SG tumours. Thus, significantly higher LI is seen in the malignant SG tumours than benign SG tumours [Table/Fig-1,2].
Descriptive statistics by Mann-Whitney U-Test for KI-67 expression between Benign and malignant salivary gland tumours
| Descriptive Statistics |
|---|
| Ranks | Test Statistics |
|---|
| groups | N | Mean Rank | Sum of Ranks | | | Ki67 |
|---|
| Ki67 | Benign | 40 | 34.70 | 1388.00 | Ki67 | Mann-Whitney U | 568.000 |
| Malignant | 50 | 54.14 | 2707.00 | Wilcoxon W | 1.388E3 |
| Total | 90 | | | | Z | -3.716 |
| | | | | | Asymp. Sig. (2-tailed) | 0.0002 |
XIAP
XIAP antigen was expressed in 10% of benign SG tumours all of with mild expression and 26% malignant SG tumours of which 61.53% were with mild expression, 30.76% were moderate and 7.69% were showing strong expression. XIAP was expressed in both epithelial cells as well as myoepithelial cells. Descriptive statistics for XIAP expression between Benign and malignant salivary gland tumours was done. Statistically significant difference was seen with p-value (p=0.016) for XIAP expression between group 1 and group 2. Thus, significantly higher XIAP expression is seen in malignant SG tumours than benign SG tumours [Table/Fig-1&3].
Descriptive statistics by Mann-Whitney U Test for XIAP expression between Benign and malignant salivary gland tumours
| Descriptive Statistics |
|---|
| Ranks | Test Statistics |
|---|
| groups | N | Mean Rank | Sum of Ranks | | XIAP |
|---|
| XIAP | Benign | 40 | 40.25 | 1610.00 | Mann-Whitney U | 790.000 |
| Malignant | 50 | 49.70 | 2485.00 | Wilcoxon W | 1.610E3 |
| Total | 90 | | | Z | -2.399 |
| | | | | Asymp. Sig. (2-tailed) | .016 |
Correlation Between Ki-67 and XIAP
Statistically significant positive correlation between Ki-67 count and XIAP expression was noted in benign and malignant SG tumours [Table/Fig-4].
Overall correlations between Ki-67 and XIAP expression in benign and malignant salivary gland tumours
| Correlations |
|---|
| | Ki-67 | XIAP |
|---|
| Ki-67 | Pearson Correlation | 1 | .614** |
| Sig. (2-tailed) | | .000 |
| N | 90 | 90 |
| XIAP | Pearson Correlation | .614** | 1 |
| Sig. (2-tailed) | .000 | |
| N | 90 | 90 |
Discussion
SG tumours are rare and the diagnosis is often not straightforward, as different types of SG tumours may share overlapping features [19]. The majority of salivary gland neoplasms are epithelial in origin with 70% being pleomorphic adenomas. Among all SG tumours, only adenoid cystic carcinoma, mucoepidermoid carcinoma and carcinoma ex pleomorphic adenoma have histologic spectrum that allows significant tumour grading [20–22]. Inspite of numerous attempts towards histopathological grading the correlation of grades with diseases progression remains ambiguous. Therefore a need is felt for specific marker indicating of aggressive nature of any particular tumour.
In the present study, evaluation of XIAP expression in 40 benign SG tumours was done of which 10% showed positive expression and in 50 malignant SG tumours, 26% showed positive expression [Table/Fig-5a-c]. The XIAP expression was localised with small number of acinar, ductal, luminal, basal and myoepithelial cells in benign tumours as compared to that of the malignant tumours [Table/Fig-5a-c]. This is found in agreement with the study by Benjamin et al., [14].
A: Photomicrograph showing XIAP expression in Pleomorphic adenoma (40X)
B: Photomicrograph showing XIAP expression in Mucoepidermoid Carcinoma (40X)
C: Photomicrograph showing XIAP expression in CXPA (40X)
The mechanisms of apoptosis are highly conserved in a plethora of species and they include a series of processes. Usually, apoptosis can be divided into three distinct phases: the initiation, the effector, and the execution phase. When outer stimuli initiate cellular apoptosis by different pathways, including death receptor-mediated and stress-dependent triggers, the imbalance between activators and IAP of apoptosis appears. After initiation, the activated caspases family and changes in mitochondrial outer membrane permeability finally result in cellular apoptosis [23]. Suppression of apoptotic process by inhibiting procaspase activation and the catalytic activity of mature caspases is mainly carried out by IAP family. As an important member of IAP family which was originally identified in baculovirus, XIAP suppresses apoptosis via inhibition on caspase-3 and -7 [24]. Thus increase in XIAP expression indicates more aggressive phenotypes.
In present study, in benign SG tumours, only pleomorphic adenoma was qualitatively graded mild [Table/Fig-5a]. In malignant tumours, maximum expression was found with CXPA which was qualitatively graded from moderate to strong; with high grade mucoepidermoid carcinoma qualitatively graded from mild to moderate [Table/Fig-5b]. CXPA was the only malignant tumour showing strong grading [Table/Fig-5c]. This was found in agreement with the study by Benjamin e al., [14]. This indicated the blocking of the downstream portion of the apoptosis pathway from multiple stimuli thereby inhibiting both the initiation and execution phases of the caspase cascade crucial to the demise of malignant cells. The other SG tumours lacking XIAP expression might be due to early stages of malignancy process thus lacking increased amount of aberrant protein and of small sample size of those particular subset. An alternative interpretation, for example, that staining heterogeneity could be, to some degree, fixation-dependent, is not categorically ruled out in the present study.
Thus XIAP expression is more in carcinomas indicating a defective apoptotic pathway in malignant neoplasm. Abnormalities in the expression of XIAP, P53, and Her-2/c-erB-2 may represent components of a complex pathogenesis involving multiple molecular pathways or may be secondary events to a single initial pathway resulting in tumour development [9–11,14,19].
Ki-67 antigen is present in all the active parts of cell cycle except G0 phase and reaches its peak during the G2 and M phases [25]. Ki-67 expression is increased with the cell cycle dysregulation. In previous some studies, Ki-67 expression was detected negative in benign SG tumours [26,27] while others noted sporadic Ki-67 expression ranging from 0.00 to 2.81 in the same [19,28]. Recently, Vargas et al., [19] noted Ki-67 LI of 8.38 (±7.24) in malignant salivary gland tumours.
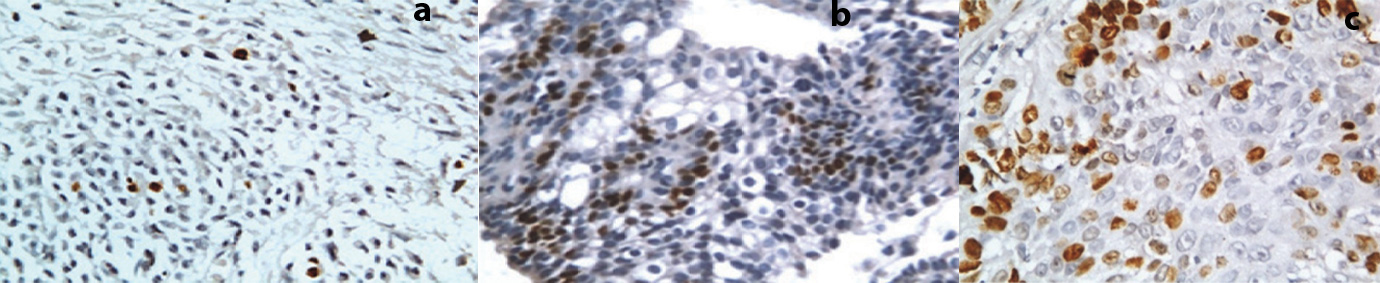
In the present study, Ki-67 expression was evaluated in 40 benign SG tumours, 40% of them showed positive expression and in 50 malignant SG tumours, 48% of them showed positive expression [Table/Fig-6a-c]. Almost all the ductal and sheet pattern of malignant epithelial cells was positive for Ki-67 expression, which was suggestive of high biological aggressive behaviour of tumour with poor prognosis. Ki-67 labelling index was increased in malignant salivary tumour as compared to benign SG tumours indicating high proliferative activity (e.g. cells committed to cell cycle). This was found in agreement to the study by Vargas et al., and Lazzaro et al., [19,26].
A: Photomicrograph showing Ki 67 expression in Pleomorphic adenoma (40X)
B: Photomicrograph showing Ki67 expression in Mucoepidermoid Carcinoma (40X)
C: Photomicrograph showing Ki67 expression in CXPA (40X)
When individual and overall correlation was drawn between XIAP and Ki-67 expression in benign and malignant salivary gland tumours, it was found to be statistically significant. Thus, this present finding not only goes in the favour as the individual function of the marker used but also when they are correlated with each other. As the expression of XIAP protein, an antiapoptotic marker increases, tumour cell death is inhibited or decreased resulting in uncontrolled cell proliferation than normal. Ki-67 one of the essential protein necessary for cell proliferation indicates that the antiapoptotic activity and proliferative activity of the tumour cells is much less in benign salivary gland lesion and is significantly much higher in malignant salivary gland neoplasm.
In general, as tumours become more undifferentiated they acquire cellular alterations that may provide survival and growth advantages and increase clinical aggressiveness. In particular, the enhanced ability to resist apoptosis could increase the likelihood of a malignant cell surviving in stressful or distant microenvironments; including exposure to radio- or chemotherapy and localization to metastatic sites [6]. This is the first study carried out in such a large cohort of salivary glands tumours and third one in oral neoplasm using XIAP marker [14,29]. Also, XIAP-targeting drugs flavonoid phenoxodiol, which has been shown to reduce XIAP and potentiate the action of chemotherapeutic agents in vitro, restored chemo responsiveness in a subpopulation of patients with recurrent ovarian carcinoma [30]. The present findings raise the possibility that XIAP expression could be one of the factors responsible for the ineffectiveness of these therapies in salivary gland tumours.
Conclusion
The study was designed using two markers that is one a proliferative and other IAP. It was anticipated that there would be direct relationship between their expression that is increase in one would cause increase in other. Both the markers showed increase expression from benign to malignant SG tumours. Both experimental and clinical studies suggest that reversal of XIAP actions may enhance therapeutic efficacy. For example, rituximab-induced decrease of XIAP protein levels has been shown to sensitize chronic lymphocytic leukaemia cells to the cytotoxic effects of chemotherapy in vivo. Reversal of radiation resistance has been demonstrated in tumour cells transfected with an adenoviral XIAP antisense vector. XIAP-targeting drugs might similarly hold promise form treatment of unresectable, widely metastatic, or drug-resistant SG malignancy. Further continuation of this study is needed for other SG tumours with large sample size for evaluating the expression of XIAP protein and its role in future target therapy.
Abbreviations
| XIAP: | X-linked inhibitor of apoptotic protein |
| IAP: | Inhibitor of apoptotic protein |
| LI: | Labelling index |
| CXPA: | Carcinoma ex-pleomorphic adenoma |
[1]. Fäbio A Alves, Daniel EC Perez, Osley P Almeida, Márcio A Lopes, Luiz P Kowalski, Pleomorphic Adenoma of the Submandibular Gland Clinicopathological and Immunohistochemical Features of 60 Cases in BrazilArch Otolaryngol Head Neck Surg 2002 128:1400-03. [Google Scholar]
[2]. Johns ME, Goldsmith MM, Incidence, diagnosis, and classification of salivary gland tumours. Part 1Oncology (Huntingt) 1989 3(2):47-56. [Google Scholar]
[3]. Ferreira Carlos G, Valk Paul van der, Span Simone W, Ludwig Inge, Smit Egbert F, Kruyt Frank AE, Expression of X-linked Inhibitor of Apoptosis as a Novel Prognostic Marker in Radically Resected Non-Small Cell Lung Cancer PatientsClin Cancer Res 2001 7:2468-74. [Google Scholar]
[4]. Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, Gabbert HE, Gerharz CD, XIAP expression is an independent prognostic marker in clear-cell renal carcinomasHum Pathol 2004 35(8):1022-28. [Google Scholar]
[5]. LaCasse EC, Baird S, Korneluk RG, MacKenzie AE, The inhibitors of apoptosis (IAPs) and their emerging role in cancerOncogene 1998 17:3247-59. [Google Scholar]
[6]. Schimmer AD, Dalili S, Batey RA, Riedl SJ, Targeting XIAP for the treatment of malignancyCell Death Differ 2006 13:179-88. [Google Scholar]
[7]. Holcik M, Gibson H, Korneluk RG, XIAP: apoptotic brake and promising therapeutic targetApoptosis 2001 6:253-61. [Google Scholar]
[8]. Lewis JE, Olsen KD, Sebo TJ, Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 casesHum Pathol 2001 32:596-604. [Google Scholar]
[9]. Freitas LL, Araujo VC, Martins MT, Biomarker analysis in carcinoma ex pleomorphic adenoma at an early phase of carcinomatous transformationInt J Surg Pathol 2005 13:337-42. [Google Scholar]
[10]. DiPalma SD, Skalova A, Vanieek T, Non-invasive (intracapsular) carcinoma ex pleomorphic adenoma: recognition of focal carcinoma by HER-2/neu and MIB1 immunohistochemistryHistopathology 2005 46:144-52. [Google Scholar]
[11]. Ohtake S, Cheng J, Ida H, Precancerous foci in pleomorphic adenoma of the salivary gland: recognition of focal carcinoma by P53 immunohistochemistryJ Oral Pathol Med 2002 31:590-97. [Google Scholar]
[12]. Altemani A, Martins MT, Freitas L, Cacinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in carcinomatous progressionHistopathology 2005 46:635-41. [Google Scholar]
[13]. Foschini MP, Gaiba A, Cocchi R, p63 expression in salivary gland tumours: role of delta Np73L in neoplastic transformationInt J Surg Pathol 2005 13:329-35. [Google Scholar]
[14]. Hoch Benjamin L, Maoxin Wu, Michael Lewis, Li Gan, David E. Burstei, An immunohistochemical study of XIAP expression in pleomorphic adenoma and carcinoma ex pleomorphic adenomaJ Oral Pathol Med 2008 37:634-38. [Google Scholar]
[15]. Muris JJ, Cillessen SA, Vos W, van Houdt IS, Kummer JA, van Krieken J, Immunohistochemical profiling of caspase signalling pathways predicts clinical response to chemotherapy in primary nodal diffuse large B-cell lymphomasBlood 2005 105:2916-23. [Google Scholar]
[16]. Tamm I, Richter S, Scholz F, Schmelz K, Oltersdorf D, Karawajew L, XIAP expression correlates with monocytic differentiation in adult de novo AML: impact on prognosisHematol J 2004 5:489-95. [Google Scholar]
[17]. Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemiaClin Cancer Res 2004 10:3737-44. [Google Scholar]
[18]. Schluter C, Duchrow M, Wohlengerg C, Becker MHG, Key G, Flad HD, The Cell Proliferation-associated Antigen of Antibody Ki-67: A Very Large, Ubiquitous Nuclear Protein with Numerous Repeated Elements, Representing a New Kind of Cell Cycle-Maintaining ProteinsThe Journal of Cell Biology 1993 123(3):513-22. [Google Scholar]
[19]. Vargas PA, Cheng Y, Barrett AW, Craig GT, Speight PM, Expression of Mcm-2, Ki-67 and geminin in benign and malignant salivary gland tumoursJ Oral Pathol Med 2008 37(5):309-18. [Google Scholar]
[20]. Ide F, Suka N, Kitada M, Sakashita H, Kusama K, Isikawa T, Skin and salivary gland carcinogenicity of 7, 12-dimethylbenz [a] anthracene is equivalent in the presence or absence of aryl hydrocarbon receptorCancer Lett 2004 214(8):35-41. [Google Scholar]
[21]. Azuma M, Tamatani T, Kasai Y, Sato M, Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori-mutant deoxyribonucleic acidLab Invest 1993 69:24-42. [Google Scholar]
[22]. Espinal EG, Uvios AM, Cabrini RL, Salivary gland tumours induced by 32 PJ Oral Pathol 1984 13:686-91. [Google Scholar]
[23]. Hockenbery DM, Giedt CD, O’Neill JW, Mitochondria and apoptosis: new therapeutic targetsAdv Cancer Res 2002 85:203-42. [Google Scholar]
[24]. Deveraux QL, Reed JC, IAP family proteins—suppressors of apoptosisGenes Dev 1999 13:239-52. [Google Scholar]
[25]. Brown DC, Gatter KC, Ki-67 protein: the immaculate deception?Histopathology 2002 40:2-11. [Google Scholar]
[26]. Lazzaro B, Cleveland D, P53 and Ki-67 antigen expression in small oral biopsy specimens of salivary gland tumoursOral Surg Oral Med Oral Pathol Oral Radiol Endod 2000 89(5):613-17. [Google Scholar]
[27]. Alves FA, Pires FR, de Almeida OP, Lopes MA, Kowalski LP, PCNA, Ki-67 and p53 expression in submandibular salivary gland tumoursInt J Oral Maxillofac Surg 2004 33:593-97. [Google Scholar]
[28]. Aoki T, Tsukinoki K, Karakida K, Ota Y, Otsuru M, Kaneko A, Expression of cyclooxygenase-2, Bcl-2 and Ki-67 in pleomorphic adenoma with special reference to tumor proliferation and apoptosisOral Oncology 2004 40:954-59. [Google Scholar]
[29]. Nagi Chandandeep, MD, Xiao Guang-Qing, MD, Li Gan, PhD, Genden Eric, MD, Burstein David E., MD, Immunohistochemical detection of X-linked inhibitor of apoptosis in head and neck squamous cell carcinomaAnnals of Diagnostic Pathology 2007 11:402-06. [Google Scholar]
[30]. Bannerji R, Kitada S, Flinn IW, Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistanceJ Clin Oncol 2003 21:1466-71. [Google Scholar]