Antimicrobial usage is like a double edged sword. These can produce life saving and disease modifying effects, on the other hand its use can result in complications like anaphylaxis, organ toxicities and development of drug resistance [1]. Since resistance to antimicrobials occurs late and is less obvious, the importance of preserving these therapeutic resources has not been fully understood. Towards this end, several studies have been undertaken and these have shown that there is a definite relationship between antibiotic usage and bacterial resistance [2,3]. However, there is very little information available regarding antibiotics and the resistance pattern of prevalent microbes in hospitals across the country.
The intensive care unit (ICU) being the epicentre of infections, use of broad-spectrum antibiotics in critically ill patients is inevitable because of little margin for error in choice of therapy [4]. Misuse and overuse of antimicrobials in intensive care units (ICUs) is of particular concern as it creates selective evolutionary pressure and thereby enables antimicrobial resistant bacteria to increase rapidly resulting in loss of antimicrobial effectiveness [5].
Studies on drug utilization in the health care setting helps in detecting early signals of irrational drug use and also serve as indicators of drug prescribing practices. Antibiotic utilization can be measured in units such as defined daily dose (DDD), antibiotic days, and cost of therapy. The WHO definition “DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults” [6]. The DDD per 100 bed-days has been used to follow changes in prescribing habits arising from the acceptance of and adherence to antibiotic policy guidelines [7,8]. An advantage of the DDD as a comparison unit is that it changes in the package size or dosage cannot distort the measured consumption.
To maximize the benefits and minimize the unwanted effects of antibiotics it is essential that they are used appropriately and adequately. An institutional approach to optimizing antimicrobial use with regards to appropriate selection, dosing and duration can become a tool to minimize the development of resistance among clinically important pathogens. This can be achieved by instituting stringent antibiotic policies in the institutions. For organizations like Society of Healthcare Epidemiology of America (SHEA) and Infectious Disease Society of America (IDSA), Centers for Disease Control and Prevention (CDC) and World Health Organization(WHO) optimizing antibiotic use has become a priority [9]. With this objective they have created guidelines on Antibiotic Stewardship to streamline the use of antimicrobials. These include two major interventions like “Prospective audit and feedback program” and “preauthorization program” [10]. In our hospital prospective audit with feedback was initiated followed by restriction on usage of antimicrobials. The antimicrobials were categorized as A, B & C. Category “A” includes primary use antibiotics which are routine for specific organisms groups. Category B includes antimicrobials that are used selectively such as when organism is resistant to Category A. Category “C” includes newer antimicrobials and those which show sensitivity to multidrug resistant organisms like Carbapenems, 4th generation cephalosporins, tigecycline, vancomycin, aztreonam, colistin, teicoplanin, voriconazole, and amphotericin B and polymixin. A Reserve Drug Indent Form (RDIF) which includes Category C drugs was introduced as a part of preauthorization program. This restricts the reserve drugs use for special cases, where in their use has been proved or justified.
Materials and Methods
Setting
This was a retrospective observational study (single site) conducted in a 20 bedded MICU of our tertiary care hospital for a period of one year between July 2012 & August 2013. Adult patients of either sex aged above 18 y admitted in the MICU and receiving reserve antimicrobial treatment for more than three days met the inclusion criteria for the study. Neonates and children below 18 y, patients admitted to coronary care unit (CCU), surgical intensive care (SICU), other specialized intensive care units and ward admissions were excluded from the study.
Intervention
Carbapenems, 4th generation cephalosporins, tigecycline, vancomycin, aztreonam, colistin, teicoplanin, voriconazole, and amphotericin B were categorized as reserve antimicrobials by the institution and “Reserve drug indent form (RDIF)” was introduced in the month of January 2013. From March 2013, reserve drugs were dispensed by the pharmacist only on receiving these forms with adequate justification by the prescribers. So, this form will serve as a basis for collecting data on the consumption of these antimicrobials [ANNEXURE].
The study period of 1 year was divided into two phases: Phase I (6 months – July to December 2012) was before introduction of the RDIF and Phase II (6 months – March to August 2013) was after introduction of the RDIF. Data regarding the AM consumption in ICU during the study period was collected from the pharmacy database.
Calculation of the rate of Antimicrobial Consumption
An antimicrobial consumption calculator (ABC Calc, version 3.1; Statens Serum Institute) which employed Anatomical Therapeutic Chemical (ATC) classification index and defined daily dose (DDD) software version 2006 was used. AM consumption for each drug was calculated as DDD/ 100 bed days.
As per the ATC/DDD classification

The occupancy index (OI) was calculated every month and was derived by dividing the number of occupied beds by the total number of beds in the ICU. Total number of beds = 20 and average number of beds occupied = 10. Occupancy index = 0.5
The total consumption of antimicrobials per month was calculated by adding all individual drugs during that particular month. Differences in the AM consumption rate before and after the implementation of RDIF were analysed.
Prevalence of Antimicrobial Resistance
Data for isolates of the following five pathogens were obtained from the hospital microbiology department: Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacterbaumannii, and Methicillin resistant Staphylococcus Aureus. These isolates were studied for sensitivity to seven reserve antimicrobials-Meropenem,Imipenem, Teicoplanin, Aztreonam, tigecycline, colistin and vancomycin respectively. Sensitivity of the isolated organisms was identified using Kirby Bauer method as per CLSI guidelines. Resistance rates for the study period was calculated by dividing the number of antimicrobial-resistant isolates by the total number of isolates. The results were expressed as percentages.
Statistical Analysis
Impact of RDIF on Antimicrobial consumption
We used Unpaired t-test —to assess the changes of antibiotic use before and after the implementation of the RDIF. SPSS, version 16.0 (SPSS), was used for analysis. P < 0.05 was considered significant
Association between the AM consumption and Resistance Pattern
Individual reserve drug consumption was studied against sensitivity, for each month before and after introduction of RDIF. The impact of RDIF on AM consumption and resultant change in the sensitivity pattern observed was presented as graphs using Microsoft excel.
Results
A total of 231 patients were admitted in MICU during the entire study period of one year. Among them, only 65 (out of 121) patients before intervention and 32(out of 110) patients after intervention met the inclusion criteria. Mean age was 50.3 ± 17.3. Average length of stay was six days.
The common clinical presentations were pneumonia (38), chronic obstructive pulmonary disease (25), multiorgan dysfunction (18), poisoning (5), cerebrovascular accident (4), renal failure on dialysis (5),gastrointestinal infection (2).There was no much difference in the demographic details and morbidities of the patients during both the periods.
Antimicrobial Use and Impact of RDIF on Consumption
The total Reserve antimicrobial consumption was 125.79 per 100 bed days: 85.55/100bed days before intervention and 40.24 /100bed days after the introduction of the indent form [Table/Fig-1].
Reserve Antimicrobial consumption during the study period-before and after implementation of RDIF expressed as DDD/100bed days
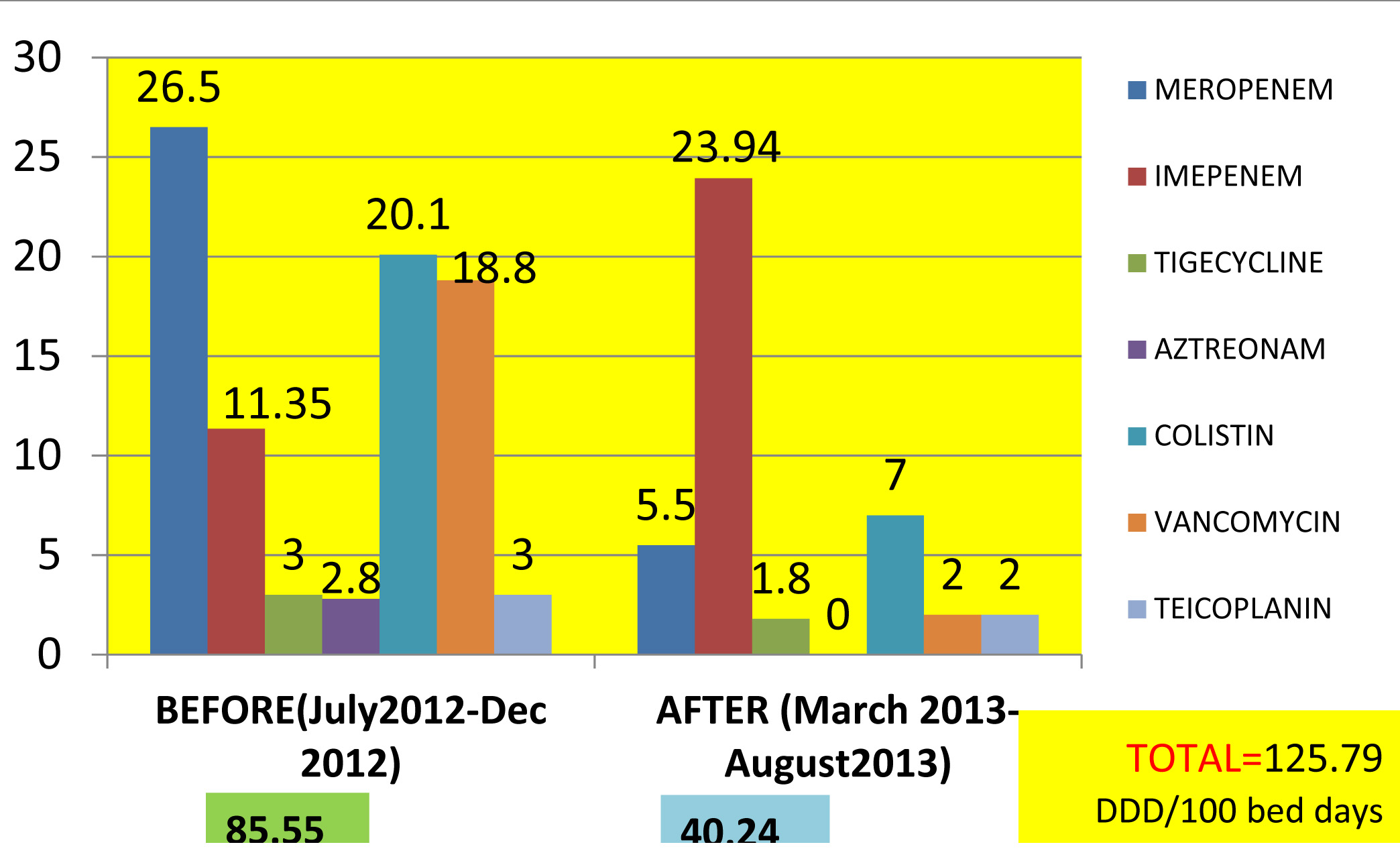
Student t-test results of p<0.05 showed that there is significant difference in drug consumption before and after implementation of RDIF with regards to meropenem, imipenem, colistin and vancomycin usage. But teicoplanin, aztreonam, tigecycline usage was low from the beginning [Table/Fig-2]
Antimicrobial usage - before and after implementation of RDIF
| Antimicrobial Usage | Before | After | p-value |
|---|
| Meropenem | 26.5 | 5.5 | 0.03* |
| Imipenem | 11.35 | 23.94 | 0.05* |
| Tigecycline | 3 | 1.8 | 0.54 |
| Aztreonam | 2.8 | 0 | 0.38 |
| Colistin | 20.1 | 7 | 0.05* |
| Vancomycin | 18.8 | 2 | 0.04* |
| Teicoplanin | 3 | 2 | 0.38 |
*p value <0.05 is significant.
Sensitivity Profile
Specimens
The total number of clinical samples received from patients was 265, of which 56 was blood, 70 urine, 80 sputum, 12 pus/wound. Bacterial growth was observed in 119(44.9%) specimens – pus 11 (9.2%), sputum 60 (50.4%), blood 18(15.1%) and urine 30 (25.2%).
Sensitivity profile of five different organisms isolated from the above clinical samples: Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacterbaumannii, and Methicillin resistant Staphylococcus Aureus, before and after implementation of the RDIF [Table/Fig-3].
Showing the sensitivity profile of 5 organisms for the Reserve AM before and after introduction of RDIF
| % Sensitivity of Strains | Acinetobacter spp. | Pseudomonas spp. | E. coli | Klebsiella spp. | Staphylococcus aureus |
|---|
| Imipenem | 79.20 | 71.4 | 85.7 | 70.4 | 78.6 | 60.4 | 84.6 | 64.7 | - | - |
| Meropenem | 65.7 | 75.5 | | | | | | | - | - |
| Vancomycin | - | - | - | - | - | - | - | - | 92 | 100 |
| Teicoplanin | - | - | - | - | - | - | - | - | 100 | 100 |
| Aztreonam | 62.5 | 61.7 | 65.2 | 66.2 | 50.0 | 57.1 | 54.6 | 50.8 | - | - |
| Colistin | 94 | 100 | 87.8 | 98.0 | 92 | 100 | 92 | 99.1 | - | - |
| Tigecycline | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 99.4 | - | - |
Black = July-Dec 2012 Red = March-August 2013
Association Between the Antimicrobial Consumption and Sensitivity
Among the more frequently used reserve drugs, meropenem, imipenem, vancomycin and colistin showed significant difference in consumption before and after implementation of RDIF. Over the 12 months study period, AMs were further analysed for association between their consumption and sensitivity.
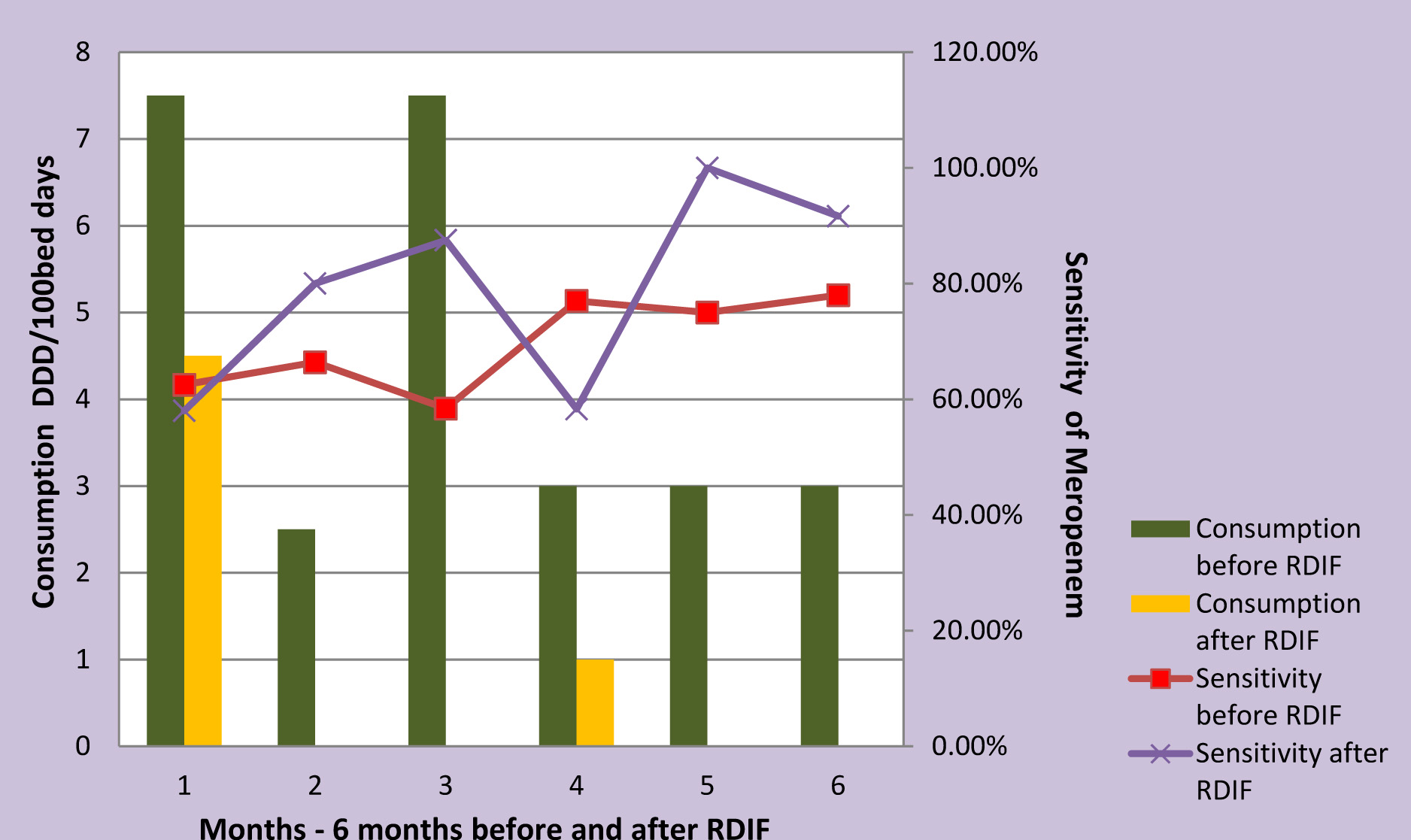
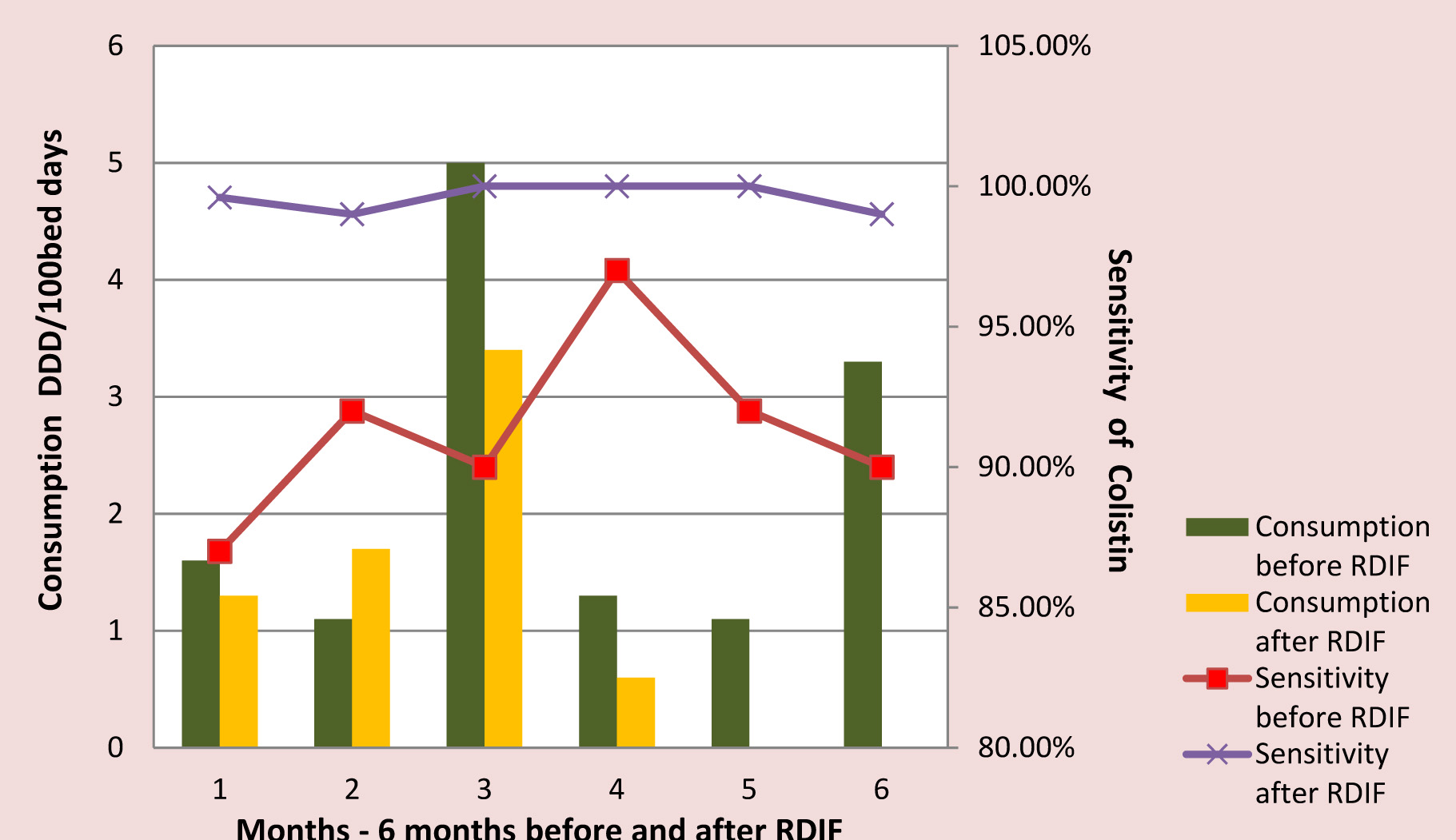
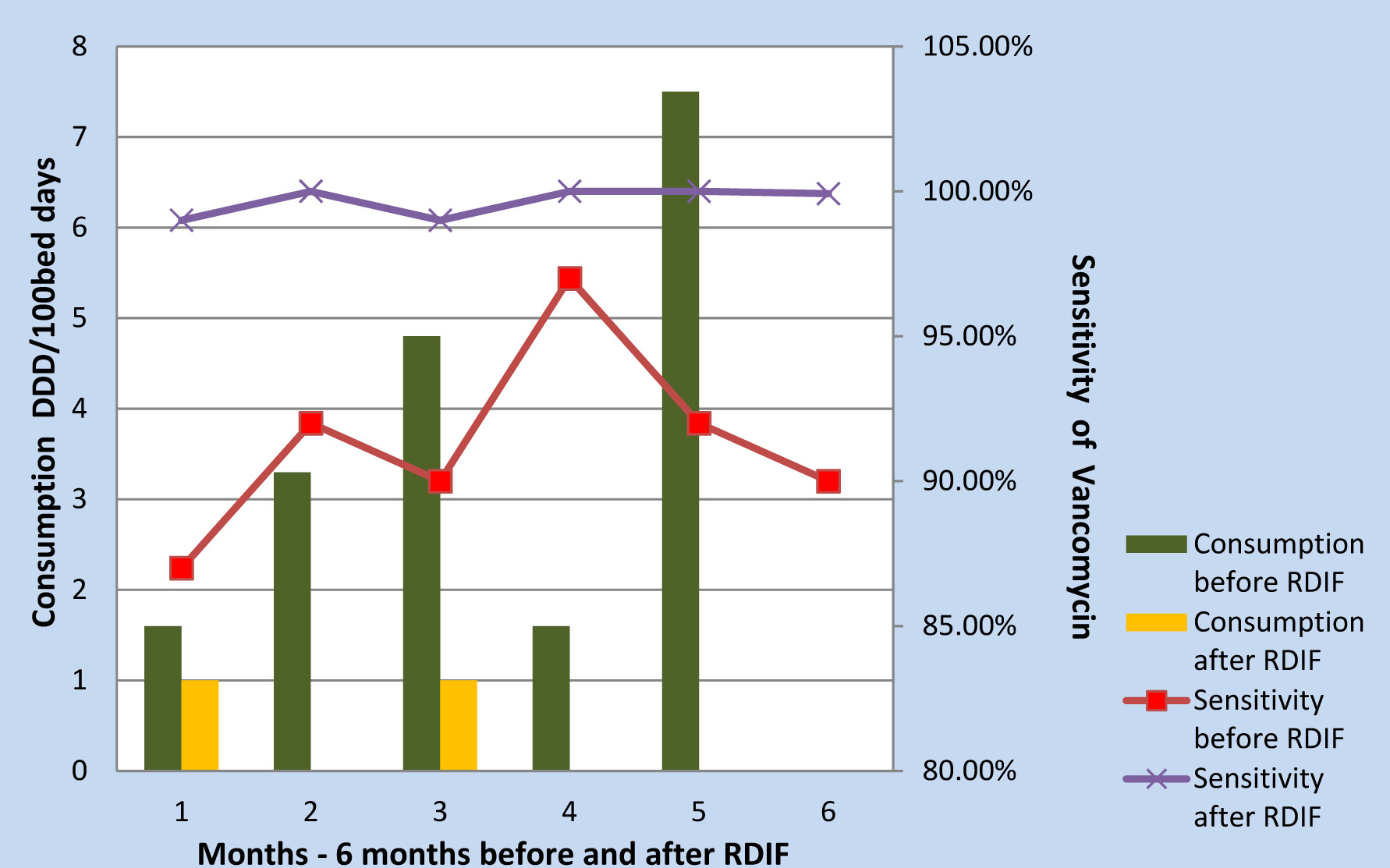
Meropenem, colistin and vancomycin consumption reduced following the implementation of RDIF simultaneously the antibiogram showed improved sensitivity [Table/Fig-4,Table/Fig-5,Table/Fig-6,Table/Fig-7,8].
Meropenem Consumption Vs Sensitivity
Colistin Consumption Vs Sensitivity
Vancomycin Consumption Vs Sensitivity
In the latter half of the study since imipenem was relatively more sensitive than meropenem, consumption of imepenem showed an increase. As a fall out of the increased usage of imepenem sensitivity reduced [Table/Fig-7].
Imipenem Consumption Vs Sensitivity
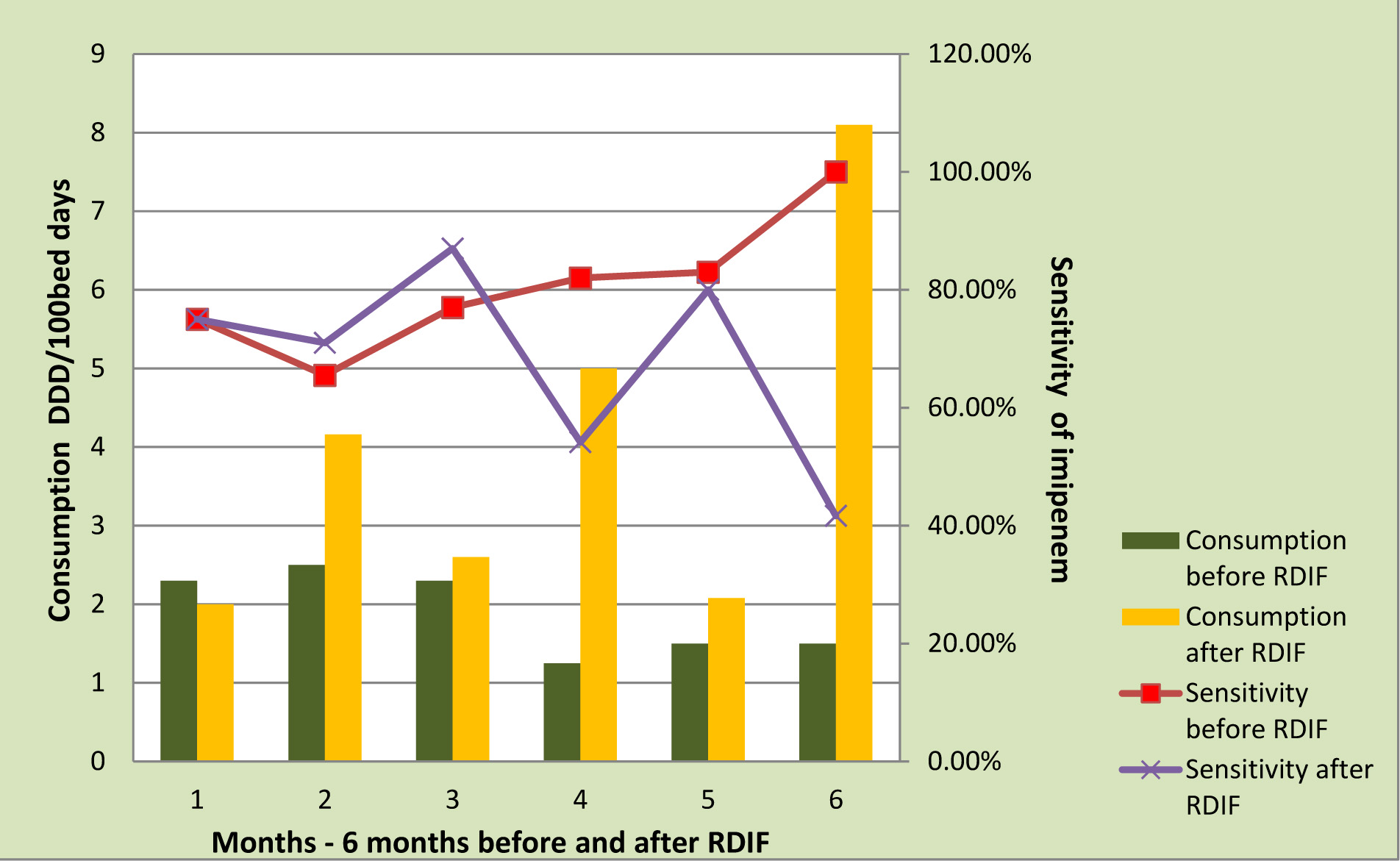
Impact on Cost
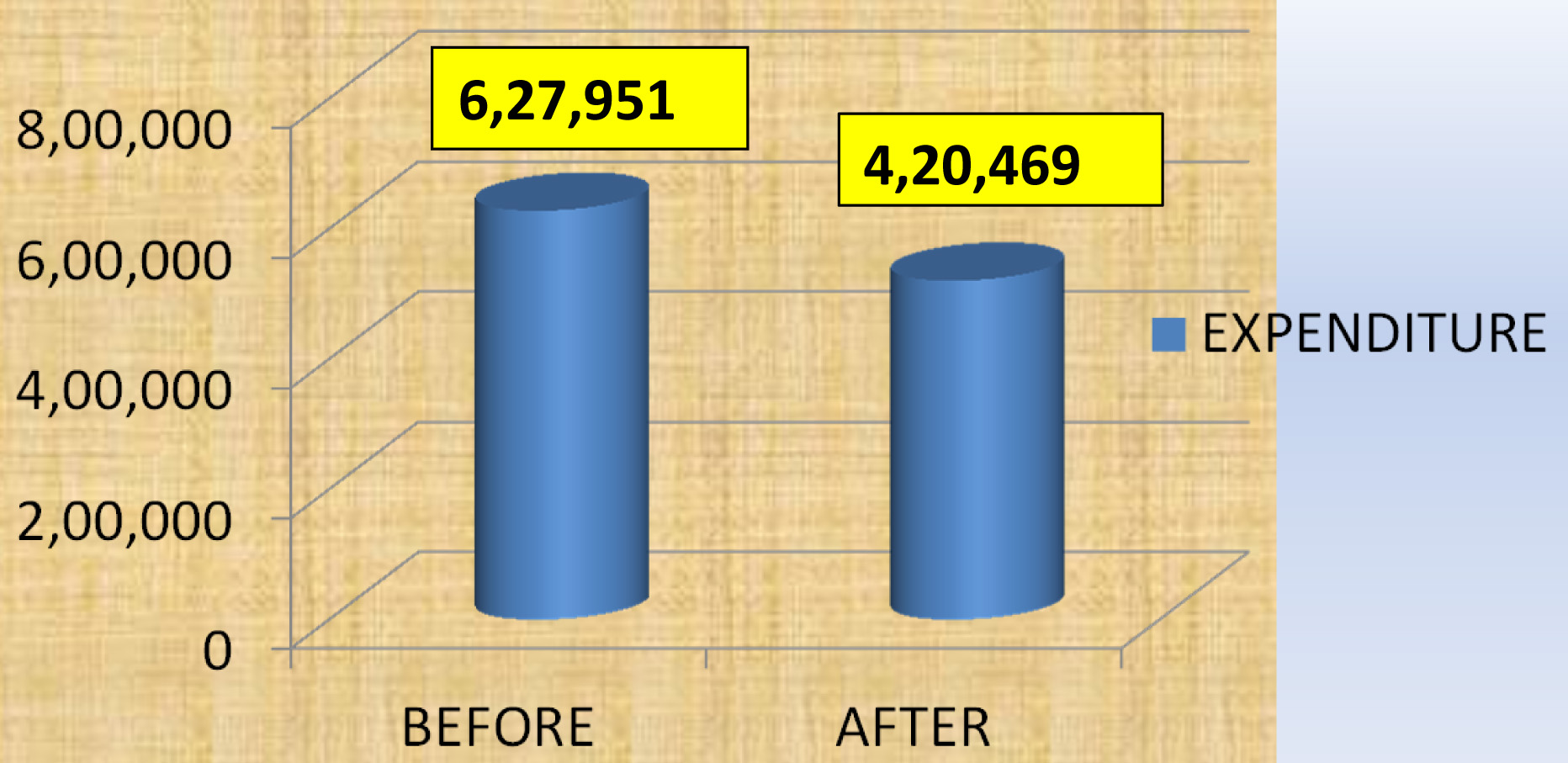
Cost of the therapy was calculated by adding up the cost of individual vials for both the periods separately. Study revealed that the total cost of therapy reduced from Rs 6, 27,951 to Rs 4, 20,469 following intervention with RDIF [Table/Fig-8].
Expenditure before and after implementation of RDIF
Discussion
Antimicrobials are the corner stone of therapy in ICU settings, but misuse, abuse and overuse of these drugs has led to the emergence of alarming levels of AM resistance. This is of great concern as it contributes to failure of therapy leading to poor prognosis, prolonged hospital stay and increased cost of therapy [11]. Therefore, to reduce the development of AM resistance regulation of their use is essential [12,13]. These regulations can be customized to suit the requirements of the institution. The Antimicrobial Stewardship Program (ASP) in our hospital follows the guidelines suggested by SHEA and IDSA. Introduction of RDIF is a part of our ASP. In this study, the trend in AM consumption before and after implementation of Reserve Drug Indent Form was evaluated. Intervention with RDIF showed, reducing trend in overall consumption of reserve antimicrobials. Meropenem, vancomycin and colistin usage significantly reduced in the second half of the study. However, Imipenem continued to be prescribed at higher rates in comparison to other reserve drugs and this can be attributed to the sensitivity profile of MDR organisms.
During the study period, the sensitivity pattern of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacterbaumannii, and Methicillin resistant Staphylococcus Aureus isolated from different specimens were studied for the reserve drugs. The AM use and concomitant sensitivity profile when studied revealed significant improvement in sensitivity in the second half. This could be due to the reduction in consumption of antimicrobials during this period. This kind of association between antimicrobial use and drug resistance has been observed in many previous studies [14–16].
White et al., had implemented ASP restricting the use of antimicrobials, where pre and post implementation groups did not statistically vary with regards to diagnosis, length of stay and survival. They concluded that benefits on post ASP implementation was improved susceptibility primarily for gram negative rods, decreased usage of broad spectrum antimicrobials and reduced cost which is similar to the findings in our study [17].
Sharma et al., conducted a prospective study for four months following the implementation of reserve antimicrobial indent form (RAMIF). Study showed that there was reduced consumption over a period of four months. In their study there was no comparison on consumption patterns of reserve drugs usage before and after implementation of RAMIF. Its impact on the sensitivity pattern was also not evaluated. Whereas in our study, the association between consumption following the introduction of RDIF and its impact on sensitivity patterns has been analysed. So our study is a more comprehensive assessment of the effectivity of such intervention [18].
Drawbacks of The Study
Small number of patients enrolled.
Study period was short. Hence, seasonal variation could not be assessed which has a greater impact on the disease profile and therefore antibiotic usage.
Conclusion
For quality management of the individual hospitals, local data for bacteria, their resistance patterns and antibiotic use are important. The present study gives a general overview on antimicrobial use and its varying trends with RDIF intervention. This vigilant approach by the Hospital Infection Control Committee (HICC) served as an important tool to combat inappropriate use of antimicrobials by creating awareness among the prescribers. It also helped in improving the sensitivity and reduced the cost burden to the patient. Hence, future studies based on similar objectives on larger sample size and of longer duration is required to strongly substantiate these observations.
Acknowledgement
I would like to thank Dr.Amitha Marla, Director, A.J. Hospital and Research Centre for granting permission to conduct this study and sharing the data on hospital policy. My sincere thanks to Dr.Sudesh Rao, intensivist for explaining the feasibility of the study. My heartfelt gratitude to Mr.Harish, Cheif pharmacist and Ms.Swarnalatha for helping me with the data on drug counsumption.
Annexure
Reserve Drug Indent Form (RDIF)
Name …………………………………………………. Age …………..…… Sex …………..…… Date……………………..
Ward …………..…… Hospital No. …………..…… D.O.A. …………..……
Reserve drugs required: Name …………………………..…………………...
Dose ………………………………………………...
Frequency …………………………………………..
Route …………………………..……………………
Signature of Nurse
Reason for choice: □ Empirical therapy
□ Prophylactic therapy
□ Antibiotic Sensitivity Test showing MDR
□ Others (specify) ………………………………………………
Probable duration: ………………………………………………………………………
Clinical diagnosis: ………………………………………………………………………
Culture report: ………………………………………………………………………….
Other tests supporting the diagnosis: …………………………………………………..
Others: ………………………………………………………………………………
Signature & Name of consultant
Criteria Reserve Drugs:
a. Critical areas: To be used as treatment in case of sepsis/unstable patients/clinically relevant cases
b. De-escalation if antibiotic sensitivity pattern shows alternate drug sensitive to the pathogen
c. De-escalation not required, if resistant to category A & B as per the Culture report.
Criteria for Reserve drugs in Non-Critical areas:
a. To be used, if resistant to category A & B as per the Culture report.
Medical Administrator Microbiologist HICC Co-ordinator Pharmacist
Categorization of Drugs
Category A: Drugs which can be prescribed by interns/PGs/consultants as first line
Category B: Drugs which will be prescribed by consultants. The administration of such drugs will be monitored by HIC and de-escalated as per the antibiotic sensitivity report
Category C: Reserve drugs
| Category A | Category B | Category C : Reserve Drugs |
|---|
| Penicillin (Benzyl, Benzathine) | Amikacin | Aztreonam |
| Amoxycillin | Cefoperazone + Sulbactam | 4rd generation cephalosporins (Cefipime, Cefpirome) |
| Amoxyclav | Doxycycline | Carbapenems |
| Ampicillin | Gatifloxacin, Gemifloxacin | Colistin / Polymyxin |
| Amoxyclox | Levofloxacin | Daptomycin |
| Ampiclox | Linezolid | Lincomycin |
| Azithromycin | Moxifloxacin | Teicoplanin |
| 1st Gen. Cephalosporins | Piperacillin + Tazobactum | Tigecycline |
| Cefazolin | Sulbactam | Vancomycin |
| Cefotaxime | | |
| Ceftazidime | | |
| Ceftriaxone | | |
| Cefixime | | |
| Chloramphenicol | | |
| Ciprofloxacin/Ofloxacin | | |
| Cloxacillin | | |
| Clindamycin | | |
| Co-trimoxazole | | |
| Erythromycin | | |
| Gentamicin | | |
| Metronidazole/Ornidazole | | |
| Norfloxacin | | |
| Piperacillin (anti-pseudomonal) | | |