Introduction: The vermiform appendix in human is considered to be a vestigial organ by most of the authors. Absence of appendix is already reported in Indian population. Whether the human appendix is performing any function is debatable but when present it can create trouble. So if there is no appendix we can escape the ill-effects of the organ. With this hope the study has been done to see whether the appendix is really going to be rudimentary or absent or not.
Marerials and Methods: Length, external diameter, number of lymphoid follicles, maximum diameter of the follicle or submucous coat, thickening of the muscle coat and seromucosal thickening of freshly removed appendix from human cadavers were seen. After fixation in 10% formal saline tissues were stained with haematoxylin-eosin stain and photographs were taken. The results had been tabulated and statistically correlated.
Result: The parameters like number of lymphoid follicles, length and diameter all are changed as per the age advancement which is strictly indicating some functional activities of the organ which is against the idea of vestigiality of the appendix.
Conclusion: Human appendix cannot be called a vestigial organ unless the functional inactivity is proved. Lymphoid changes which occur after birth to provide the gut immunity is needed to be proved by further studies. There might be incidental absence or rudimentary appendix in human body, but that does not indicate that we would not have any appendix in future.
Introduction
From the very beginning of the modern medical science evolution the human vermiform appendix is considered as one of the most debatable topic in our body. The role of the organ in human body and its existence is really helpful for the gut protection, is yet to be decided. Agenesis of the human vermiform appendix have been reported by several authors [1,2], the ratio is 1:1,00,000 [3].
According to the evolutionary knowledge of comparative anatomy, many organs in human body can be categorized as vestiges. Vestigial in that sense that the organ seemingly has no functions or useless now but they might have functions previously which are lost someway due to evolution. The vermiform appendix is considered such an organ. Before calling the human appendix vestigial its functions should be discovered. In view of its rich vascularity and histological differentiation, the appendix is probably a specialized rather than a degenerate or vestigial structure [4]. It is very difficult to stamp appendix as a vestigial organ from several facts regarding the histomorphology, function and development of the appendix [5,6]. So now the question can arise whether the human vermiform appendix is really a vestigial organ considering some facts retrospectively which are already proved.
The human appendix may be considered as a vestigial organ as it has been proved that the removal of the organ after infancy does not create any harm [1-3]. But the appendix has developed to the extreme in human and strategically placed to an important site at the junction of midgut and hindgut. Vermiform appendix is present only in rabbits, but not in other herbivores like horses, monkeys, dogs and cats favouring a mixed to carnivores diet though they can lead a normal life [5,7]. The rabbit’s appendix is the site of lodgement of gut associated lymphoid tissue (GALT) but in human GALT is distributed mainly in Peyer’s patches. There are important differences in lymphoid structure, T-cell distribution and immunoglobulin density between human appendix and the GALT [8].
The relation in between the local immune reaction and the inflammatory bowel diseases or autoimmune diseases is under investigation now. Prof. Bill Parker gave his hypothesis that the appendix serves as a “Nature reserve” for beneficial bacteria in our gut. During a severe gut infection like in cholera this bacterial flora is lost or depleted. According to Prof. Parker the appendix allows them to be restored [9].
The appendix is sub optimally designed and actually maladaptive in human. It is already proved that without the appendix we can live a normal life [1]. It appears that the most critical part of the G.I tract in human, the vermiform appendix whether performs any functions or not but can create trouble by producing appendicitis, carcinoid tumour etc. It is now proved that the appendix can help in the formation and flare up of ulcerative colitis (U.C) [10]. Appendectomy in early age specially before the age of 20 y can reduce the risk of inflammatory bowel disease likeU.C [11]. In U.C patients the CD4+/CD8+ is significantly increased and the proportion of CD4+,CD69+ T cells significantly increased but will become reverse after four weeks of appendectomy though temporary [12].
The appendix varies from 2-20 cm in length. It is longer in children and may atrophy or diminish after mid-adult life [4]. The diameter of the appendix is due to its lymphoid tissue in the mucous and sub mucous coat. The number of lymphoid follicle is increased when the organ is active.
So now it can be said that in human if the vermiform appendix is active, then the length, the diameter and lastly the number of lymphoid follicles will be increased. These three parameters are used in the present study to have an idea whether the appendix is really going to be absent or rudimentary with evolution in human being of Indian population so that we can escape the ill effects of appendix in future.
Materials and Methods
The vermiform appendices along with caecum were collected from the fresh cadavers of Indian population. Seventy-six cadavers, 29 female & 47 male were taken during the study period from March 2008 to May 2014 (duration six years two months). To get the actual measurement the meso appendix was removed to measure the length (from the base to the tip of the appendix). Diameters of the appendix were measured at the base, at the tip and in between the two, to see the maximum diameter. The maximum diameter had been noted at the base in the present study and the findings were statistically correlated at that level. Length and the maximum diameter of appendix were measured by Vernier calliper and expressed in centimetre and millimetre respectively. After measurement all the specimens were fixed by immersion in 10% formaldehyde solution for 48 h. Tissues were collected at three levels, the base, the tip and in between the two. Each sample were taken and then processed for paraffin embedding and sectioning. Proper microscopic demonstration in the intact and straight form, each section was processed by standard protocol and sectioning at 5 mm on rotary microtome. Serial sections were prepared from each sample, out of which one was stained in haematoxylin and eosin following the standard procedure of staining. Each slide was then examined carefully under microscope to see the number of lymphoid follicles, maximum diameter of lymphoid follicle or maximum thickening of submucous coat, maximum thickness of seromucosal and muscle coat, and then photographs were taken. The specimens with gross deformities or pathology were excluded from the study.
Study had been done on random basis and results were analysed under three age groups <20yrs (Category 1), 20-40 years (Category 2), >40 years (Category 3) for both genders. For analysis of data, different soft-wares were used like Statistica version 6 (Tulsa, Oklahoma: StatSoft Inc., 2001 for descriptive study to get the results in a compact form), MedCalc version 11.6 (Mariakerke, Belgium: MedCalc Software 2011 to verify the results of comparison), GraphPad Prism version 5 (San Diego, California: GraphPad Software Inc., 2007 for comparison between the parameters).
As the variables (length, external diameters, no. of lymphoid follicles) were showing skewed distribution by Lilliefor's test of normality, analysis were done by Mann-Whitney U-test and Kruskal-Wallis test considering the data as nonparametric variables. P-value <0.05, rho (ρ) >0.300 were considered as statistically significant.
Results
Statistical analysis of the parameters of the entire study group (n=76, male=47, female=29) has been done and is presented in [Table/Fig-1]. Descriptive statistics of numerical variables among male and female groups shows slight differences, length is more in case of female specimens in age category 2 and diameter of appendix and no. of lymphoid follicles are more in case of male specimens in age category 3 and 1 which is represented by bar diagram [Table/Fig-2] But comparative study of three parameters (length, external diameter and no. of lymphoid follicles) between entire male and female by Mann-Whitney U-test showing no significant statistical differences (p-value for length, diameter &lymphoid follicles are 0.5, 0.23 &0.19 respectively).
Descriptive analysis of mean length and diameter and average no of lymphoid follicles of three different age categories has been done separately and the comparison are shown in [Table/Fig-3]. Comparison of numerical variables between three different age categories are done by Kruskal-Wallis(K-W) test followed by Dunnet’s (Dunn’s) test for post hoc comparison if Kruskal-Wallis test returns p-value < 0.05 [Table/Fig-4]. By K-W test significant differences are found between different age categories in case of length (p=0.002) and no. of lymphoid follicles (p=0.002) at the base. Dunn’s test showed that there were significant change in length of appendix among category 2 &3 age group (p value <0.01), in case of no of lymphoid follicles there is significant difference among category 1 &3 age group (p value<0.01) and category 2 &3 age group (p<0.05) irrespective of gender. No significant differences found in case of diameter in all age groups
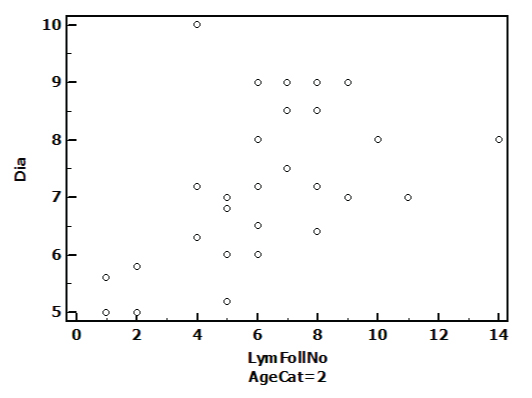
While analysing relationship among different parameters, separately in males and females we found correlation among length and no of lymphoid follicles in females only, rho(ρ)=0.38 at the base. After analysing relationship in different age categories we found that in category 1 age group correlation exist among length &no of lymphoid follicle (ρ=0.466), in category 2 age group strong correlation is existing among diameter &no of lymphoid follicle there (ρ=0.524) which is expressed in scatter diagram [Table/Fig-5]. In category 3 no correlation could be established among 3 parameters.
Discussion
At the sixth week of development a small conical dilatation called caecal bud is formed at the caudal limb of primary intestinal loop and the distal end of the caecal bud forms a narrow diverticulum, the appendix [13]. In human, the lymphoid tissue begins to appear two weeks after birth and reach a peak between the second and third decades of life; decreasing rapidly thereafter and practically disappear after sixty years of age [14]. The lymphoid tissue extending from the mucosa to submucosa frequently exhibits germinal centres within its follicles indicative of B cell activation, as occurs in secondary lymphoid tissue elsewhere. Lymphoid follicles gradually accumulate over the first decade of life to become the prominent feature of appendix. In adults normal architecture of appendix is lost due to the atrophy of the lymphoid follicles and replacement of the follicles by the collagenous tissue. In the elderly the appendix may be filled up by fibrous scar tissue [4]. The qualitative changes of the appendix in neonate most probably occur after birth due to the essential entry of certain bacteria to reside in colon during the critical stage of development [15]. The microbial biofilm distributed preferably in the large bowel is a hallmark of immune support for the microbial flora in wide range of mammalian species including frogs suggesting the adaptations supporting the biofilm growth by commensal bacteria is more ancient [16]. It has been shown that during the early years of development the appendix can perform as lymphoid organ assisting in the maturation of the lymphocytes and in production of the IgA antibodies. It is also thought that appendix can direct the movement of lymphocytes to other various locations of body by secretion of specialized molecules [17]. When the vermiform appendix is active or performing some functions, then the external diameter, number of lymphoid follicles and the length should be increased as usual. In the present study the maximum diameter of the appendix has been taken and it has been seen that the maximum diameter is at the base of the organ which is not corroborative with the work of Gupta et al., [14]. The external diameter of the appendix normally depends on lymphocyte aggregation, number of the follicles and size of the follicles. At three levels seromucosal thickenings have been measured and maximum diameters of the lymphoid follicles along with the muscle thickness have been seen to compare primarily therole of muscle coat and lymphoid follicles in the determination of the seromucosal thickenings. In the present study it is very clearly evident that the diameters of the lymphoid follicles or thickening of submucous coat have a great role in determination of the external diameters of the vermiform appendix. It is very clearly noted that the diameter of the lymphoid follicle (single largest follicle at this level) or submucosal thickening (with numerous small follicles at all levels) is more compared to muscle coat thickening. So if the diameter of the lymphoid follicle or the submucous coat with the lymphoid follicles is increased, the seromucosal thickening as well as external diameter will also be increased [Table/Fig-1]. From present study it has been seen that presence of good number of lymphoid follicles having comparatively large diameter indicating good immune response in the organ. At the same time it is also noticed that in spite of only two or three follicles some specimens are having greater diameter or there may be single follicle but it is large. In the specimen where the diameter is less either there is less number of lymphoid follicles of small size or the lymphocytes are scattered. It is very significantly noted in the present study that mean external diameter is maximum in age category 3 (>40 y) while average no. of lymphoid follicles is less. This proves that several other factors are also responsible for alteration of external diameter of appendix like wall thickness, edema of the organ or inflammatory reaction like appendicitis etc. Gupta et al., [14] in their study has found maximum diameter in <15 y of age group (8.7mm) which is not matched with our observation.
Regarding the length, authors’ observation is also not corroborating with conventional theory. They found maximum length in age category 2 which is close to the study by Galalipoir et al., [18] but again contradicting the result of Gupta et al., [14]. Due to the functional activity performed by the organ, the length is increased up to 40 y then starts decreasing due to fibrosis of the organ, thus explaining our observation about length [Table/Fig-3]. By statistical analysis (K-W test &Dunn’s test) significant differences has been found regarding length among age category 2 &3 (p<0.01) [Table/Fig-4].
To see the activity of the organ not only the diameter and length of appendix is sufficient, but also the size and number of the follicles present in it should be observed. Authors have found more lymphoid follicles at age below 20 y, especially in male [Table/Fig-6]. In age category 2, follicle are less in numbers in compare to age category 1 [Table/Fig-7] and number of lymphoid follicles are either very less or the lymphocytes are scattered at age category 3 [Table/Fig-8]. Statistical analysis regarding number of lymphoid follicle [Table/Fig-4] shows there is significant difference between age category 1 and 3 (p<0.01) and between age category 2 and 3 (p<0.05). So, from present study it is clear that there is decreasing number of follicles with increase in age. The probable explanation is that, the gut immune response is high in early age group as it is facing the foreign organisms initially. So the lymphocytes are aggregated to form the lymphoid follicle which can be found in maximum number in lower age group and as the age advances there is less number of follicles, most probably due to the settlement of the immune response of the gut. Ultimately above the age of 40 y it decreases rapidly, becomes lowest in number as the age advances more. The less number of follicles is most probably due to the merging of the small follicles in the absence of newer follicle formation in the healthy environment of the gut. Authors have found some unusual presentation in age category 3 like complete absence of lymphoid follicle or presence of more follicles [Table/Fig-9] . Presence of numerous follicles above 40 y age group may be due to any persisting inflammation of the gut. On the other hand absence of follicle or presence of only scattered lymphocytes in the older age group is due to less role of immune response of appendix to gut immunity, there by indicating the chances of appendicitis will also be less in older age group.
By analysing the associations between three parameters, authors have found no correlation is present in appendix of males but in females, length and no of lymphoid follicles is correlated, rho (ρ) being 0.38. By further analysis it is seen that in 20-40 y age group (category 2) strong correlation is present among diameter and no of follicles (ρ=0.524) whereas in category 1(<20 y age) correlation is between the length and no. of follicles (ρ=0.466). Authors have found that most probably the females of middle age group (age category 2) are much more susceptible to appendicitis because of highly active appendix which is determined by a good number of lymphoid follicles and its maximum length [Table/Fig-1]. Another very important point emerged from the study that for the radiological diagnosis of the appendicitis, consideration of the only external diameter or maximal outer diameter (≥6 mm) and mural thickness (less than 3 mm in children is normal) [19] may not be sufficient or be wrong. So for the accurate diagnosis the number of lymphoid follicles or size of the follicles should be corroborated with the diameter of the appendix which is only possible by the histomorphometric study.
It would be very much interesting to see the histomorphological changes of human appendix after birth by strictly preventing the gut from foreign organisms if possible. The study might be a complete one if the lymphocytes of the vermiform appendix also be studied to detect the histological difference of these cells from other portion of gut and also detecting the type and amount of secretion if any from the human appendicular lymphocytes.
Statistical analysis of the parameters of the entire study group (n=76, male=47, female=29)
| Base | Middle | Tip |
|---|
| Mean± SD | CI | Mean± SD | CI | Mean± SD | CI |
|---|
| UL | LL | UL | LL | UL | LL |
|---|
| Maximum(Max) Follicle diameter (micrometer) or submucous coat thickening | 92.1 ± 22.4 | 136.9 | 47.3 | 84.3 ±19.4 | 123.1 | 45.5 | 79.4 ±18.9 | 117.2 | 41.6 |
| Max. Muscle thickness (micrometer) | 58.6 ± 18.1 | 94.8 | 22.4 | 62.5 ± 17.9 | 98.3 | 26.7 | 66.8 ±17.7 | 102.2 | 31.4 |
| Max. Sero-mucosal thickness (micrometer) | 213.4 ±60.5 | 244.4 | 182.4 | 202.6 ±57.1 | 316.8 | 88.4 | 198.5 ±57.2 | 312.9 | 84.1 |
| Max. External diameter (millimetre) | 7.3 ±1.4 | 6.98 | 7.62 | 6.5 ± 1.3 | 9.1 | 3.9 | 5.8 ± 1.3 | 8.4 | 3.2 |
| Follicle number (average) | 6 | 4 | 3 |
Bar diagram showing comparison of the parameters among males and females
L- Length of appendix expressed in centimeter, D- External Diameter of appendix expressed in millimeter, LF- Number of Lymphoid Follicles
Age cat1- <20yrs, Age cat2- 20yr-40yr, Age cat3 - >40yr
Comparative study of parameters in 3 different age groups
Age category 1- <20yrs, Age category 2- 21yr-40yr, Age category 3 - >40yr
| Parameters | Age category 1(n=14) | Age category 2(n=31) | Age category 3(n=31) |
|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
|---|
| Length (cm) | 7.98 | 7.48 8.48 | 8.72 | 8.10 9.33 | 7.57 | 7.23 7.91 |
| External Diameter (mm) | 6.78 | 6.23 7.32 | 7.3 | 6.80 7.79 | 7.54 | 6.95 8.12 |
| No. of lymphoid follicle (average) | 7 | 6 | 4 |
Comparison of numerical variables between three different age categories
Len 1, Len 2, Len 3 - Length of age category 1, 2, 3. Lym foll No 1, 2, 3 – Number of lymphoid follicle in age category 1, 2, 3. ns - Statistically non significant
| Parameters | Kruskal-Wallis (K-W) Test values | Dunn’s Test value |
|---|
| H | p | Age categories | p-value |
|---|
| Length | 12.114 | 0.002 | Len1 vs Len2 Len1 vs Len3 Len2 vs Len3 | 4.563(ns) 1.095(ns) 0.008(<0.01) |
| Diameter | 2.48 | 0.289 | -- |
| No. of Lymphoid Follicle | 12.79 | 0.002 | Lym Foll No1 vs Lym Foll No2 Lym Foll No1 vs Lym Foll No3 Lym Foll No2 vs Lym Foll No3 | 2.761(ns) 0.006(<0.01) 0.023(<0.05) |
Scatter-gram depicting relationships between diameter and lymphoid follicles in Age category 2
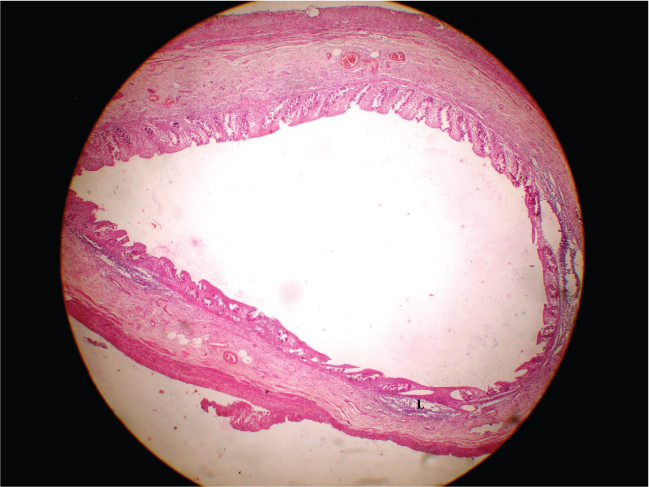
Photograph of H/E stained section of human appendix of a 16 years (age category 1) female having diameter of 9mm and lymphoid follicles (L) no. 11
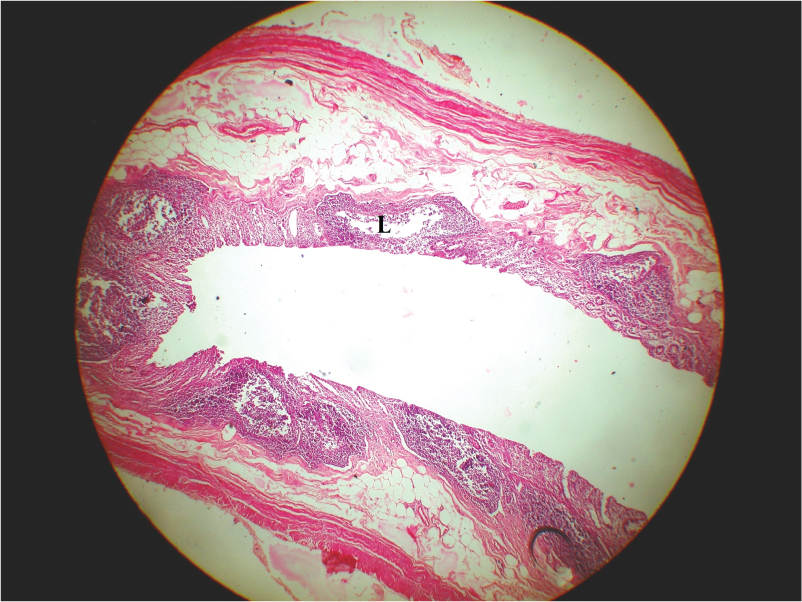
Photograph of H/E stained section of human appendix of a 35 years (age category 2) male, having diameter of 7mm and lymphoid follicles (L) no. 7 (age category 1)

Photograph of H/E stained section of human appendix of 55 years (age category 3) female having diameter of 9mm and lymphoid follicles (L) no. 3

Photograph of H/E stained section of human appendix of 80 years (age category 3) male having diameter of 6mm and lymphoid follicles (L) no.11

Conclusion
Incidentally the v. appendix may be absent or rudimentary in our body but when present it is certainly performing some functions most probably immunological which is not clear till now. The histomorphological changes after birth of the human appendix is simply a response towards the inflammation of gut or through the response it is performing some role in gut protection is yet to be concluded. From the present study it can be said that the human appendix is highly sensitive to the gut immunity up to the middle year age group but the role is decreasing with advancement of age. So, it is very difficult to say that the human vermiform appendix is vestigial from the very beginning just after birth but it is going to be vestigial in older age group in normal immunological condition of the gut. A single case of rudimentary appendix or incidental finding of absence of appendix may raise the hope that we would not have to suffer from appendicitis in future but from the present study it is becoming difficult to imagine that day when there should not be any appendix in human body really.
[1]. A Sarkar, Congenital absence of the vermiform appendixSingapore Med J 2012 53(9):e189 [Google Scholar]
[2]. EL Hei, Congenital absenece of the vermiform appendixANZ J Surg 2003 73:862 [Google Scholar]
[3]. F Chevre, M Gillet, H Vuilleumier, Agenesis of the vermiform appendixJournal: Surgical laparoscopy, endoscopy & percutaneous techniques 2000 10(2):110-12. [Google Scholar]
[4]. S Standring, NR Borley, P Collins, RA Crossman, MA Galzoulis, JC Healy, Caecum, vermiform appendix and ascending colon: large intestine. In: Standering S, ed. Gray’s Anatomy: The Anatomical Basis of Clinical PracticeChurchill Livingstone Elsevier 2008 40th EditionChina:1142-44. [Google Scholar]
[5]. BK Malla, The study on “Vermiform Appendix”- a caecal appendage in common laboratory animalsKathmandu University Med J 2003 1(4):272-75. [Google Scholar]
[6]. GB Scott, The Primate caecum and appendix vermiformis: a comparative studyJ Anat 1980 131(Pt3):549-63. [Google Scholar]
[7]. J Shoshani, CP Groves, E Simons, Primate Phylogeny. Morphological versus Molecular resultsMolPhylogenetEvol 1996 5:102-54. [Google Scholar]
[8]. JF Dasso, MD Howell, Neonatal appendectomy impairs mucosal immunity in rabbitsCell Immunol 1997 182:29-37. [Google Scholar]
[9]. RR Bollinger, AS Barbas, EL Bush, SS Lin, W Parker, Biofilms in the large bowel suggest an apparent function of the human vermiform appendixJ Theor Biol 2007 249:826-31. [Google Scholar]
[10]. RE Andersson, G Olaison, C Tysk, A Ekbom, Appendectomy and protection against ulcerative colitisN Engl J Med 2001 344:808-14. [Google Scholar]
[11]. N Buergel, JD Schulzke, M Zeitz, Appendectomy reduces the risk of development of ulcerative colitisChirurg 2002 73:805-08. [Google Scholar]
[12]. SW Kim, Clinical Improvement of severe ulcerative colitis after incidental appendicectomy-a case reportKorean J Gastroenterol 2006 47(6):463-66. [Google Scholar]
[13]. TW Saddler, Langman’s Medical Emmbryology: Digestive system 2004 PhiladelphiaLippincott Williams and Wilkins:308-09. [Google Scholar]
[14]. G Gupta, SK Srivastava, SK Mathur, Histomorphometric characteristics of human vermiform appendix with special reference to lymphoid tissueJ Morphol Sci 2012 29:135-39. [Google Scholar]
[15]. M Goodman, CA Porter, J Czelusniak, Toward a phylogenetic classificationof primates based on DNA evidence complimented by fossil evidenceMolPhylogenetevol 1998 9:585-98. [Google Scholar]
[16]. JF Dasso, H Obiakor, AO Anderson, RG Mage, A morphological and immunohistologicalstudy of the human and rabbit appendix for comparison with the avian bursaDev Comp immunol 2000 24:798-814. [Google Scholar]
[17]. LG Martin, What is the function of human appendix? Did it once have a function that has since been lost 1999 http://www.scientificamerican [Google Scholar]
[18]. MJ Galalipoir, B Arya, R Azarhoosh, M Jahanshatu, Anatomical variations of vermiform appendix in south-east Caspian SeaJ Anat Soc India 2003 52:141-43. [Google Scholar]
[19]. NH Park, HH Oh, HJ Park, Ultrasonography of normal and abnormal appendix in childrenWorld J Radiol 2011 3(4):85-91. [Google Scholar]