Evidence of Paternal N5, N10 - Methylenetetrahydrofolate Reductase (MTHFR) C677T Gene Polymorphism in Couples with Recurrent Spontaneous Abortions (RSAs) in Kolar District- A South West of India
Shiny Vanilla1, C.D. Dayanand2, Pushpa F Kotur3, Moideen A Kutty4, Pradeep Kumar Vegi5
1 Lecturer, Department of Anatomy, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and ResearchKolar, Karnataka India.
2 Professor, Department of Biochemistry/ Head, Allied Health Sciences, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka India.
3 Professor & Head, Department of Obstetrics and Gynecology, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka India.
4 Professor, Department of Biochemistry/ Dean, Allied Health Sciences, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka India.
5 Senior Research Fellow/ Research Scholar, Department of Cell Biology and Molecular Genetics/ Biochemistry, Sri Devaraj Urs Academy of Higher Education and Research Kolar, Karnataka India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Dayanand C.D, Professor, Department of Biochemistry/ Head, Allied Health Sciences Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and Research Kolar, Karnataka India.
E-mail: cd8905@yahoo.co.in
Introduction: Recurrent spontaneous abortion (RSA) is a multifactorial clinical obstetrics complication commonly occurring in pregnancy. Many research studies have noted the mutations such as C677T in N5, N10 - Methylenetetrahydrofolate reductase (MTHFR)gene which is regarded as RSA risk factor. This study was carried out to determine the occurrence of frequency of C677T of the MTHFR gene mutations with RSA. Aim: The purpose of present study is to determine the frequency of MTHFR C677T polymorphisms in couples with recurrent pregnancy loss and the impact of paternal polymorphisms of MTHFR C677T in recurrent pregnancy loss in population of couples living in Kolar district of Karnataka with RSA.
Design: A total of 15 couples with a history of two or more unexplained RSA were enrolled as subjects in the study and a total of 15 couples with normal reproductive history, having two or more children and no history of miscarriages were enrolled as controls.
Materials and Methods: DNA extraction from samples case and control group couples and its quantification by Agarose gel electrophoresis, assessment of DNA purity, MTHFR C 677T gene mutation detection by PCR-RFLP method.
Statistical analysis: Carried out by web based online SPSS tool.
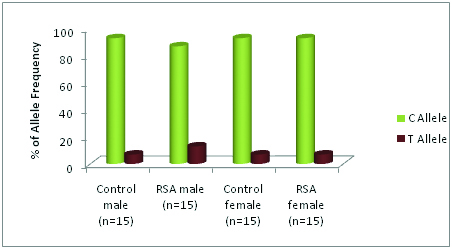
Results: The frequency of C677T genotype showed homozygous wild type CC (80%), heterozygous CT type (13.3%) and homozygous mutation TT type (6.67%) observed in males. Similarly from female’s homozygous wild type CC (86.6%), heterozygous type (13.3%), and homozygous type mutations TT (0%) was recorded. In couple control groups, we observed homozygous wild type CC (86.6%), heterozygous CT type (13.3%) and homozygous type mutations TT type (0%).
Conclusion: We noticed a high frequency of MTHFR specifically T allele associated with paternal side.Therefore, the present study indicated the impact of paternal gene polymorphism of MTHFR C677T on screening in couples with recurrent pregnancy loss.
MTHFR C677T, MTHFR A1298C, Paternal polymorphism, RSA couples, T-allele
Introduction
RSA is a multifactorial distressing disease defined as the miscarriage of two or more consecutive pregnancies before 20 weeks of gestation and affects approximately one percent of fertile couples with a significant clinical problem [1]. World Health Organization defined miscarriage as loss of the fetus or embryo weighing less than 500g, which would normally be at 20-22 complete weeks of gestation. However, in recent times, RSA is considered as the spontaneous loss of two or more consecutive pregnancies before 20 wk of gestation [2]. The RSA affected couples undergo psychological and emotional mental distress [3,4].
As per the report of International Federation of Gynecology and Obstetrics, an overall fetal wastage 10-12% on couples by means of clinically recognized or unrecognized pregnancies results with miscarriage [5]. There is a direct relationship between recurrent risk and the number of abortions. The percentage of recurrent risk for women with at least one abortion is 24%, with two abortions are 26% and three abortions is 32%. Nearly 40-45% of recurrent risks are reported in women with no live birth, infants and with two or more fetal losses [6].
Several factors are associated with RSA in terms of maternal or fetal or paternal gene polymorphisms. The various factors affecting the maternal polymorphism are genetic causes, connective tissue disorders, hematological abnormalities, anatomical and endocrine abnormalities. Similarly, the fetal causes of RSA are Autosomal dominant lethal skeletal dysplasias, Autosomal recessive disorders and X-linked Disorders [7]. Karyotyping of couples with RSA has revealed that parental chromosomal abnormalities occur in one of the partners with approximate 5 to 7% of couples [8] from where half of the cases remain unexplained. Currently, there are only limited data on a possible male cause. Hence, parental cytogenetic karyotype screening in RSA couples probably fetches the useful scientific information for identifying the gene polymorphisms.
MTHFR is one of the main regulatory enzymes in the metabolism of homocysteine that catalyses the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate [9]. Mutations in the MTHFR gene lead to decreased activity of enzyme and hyperhomocysteinemia, which induces platelet aggregation through promotion of endothelial oxidative damage [10]. Although several mutations within the MTHFR gene were described, C677T and A1298C mutations are the two most common mutations [11]. MTHFR C677T gene polymorphism is associated with fetal loss at early stages of pregnancy [12].
As a consequence of homozygous C677T polymorphism missense mutation that leads to hyper homocystenemia due to lack of MTHFR activity. However, a second polymorphism in the MTHFR gene “A1298C” results polymorphic variant inducing a decrease in MTHFR activity. The C677T polymorphism occurs in exon 4 and is characterized by an alanine-to-valine substitution at codon 222, whereas the A1298C polymorphism occurs in exon 7 and is characterized by a glutamate-to-alanine substitution at codon 429 by transverse mutation. So, it has no impact on plasma homocysteine levels, because elevated levels of homocysteine that causes vasculopathy in the placental vasculature and leads to spontaneous abortions [13].
In inherited defects within the methionine-homocysteine pathway, such as MTHFR C677T gene polymorphism, hyperhomocysteinemia and TT genotype have been associated with preeclampsia, spontaneous abortions etc. Compound heterozygosity for C677T that is 677C/T will have a similar clinical impact as C677T/T homozygosity and individuals with the 677 T/T genotype is always reported with 1298 A/A genotype and vice versa [14].
Occurrence of about 2-5% of the genetic cause of RSA is due to chromosomal abnormalities. However, the majority is unexplained with respect to RSAs. RSA affects 1-3% of couples. So far, screening procedures performed only in the women of affected couple. Studies were done at the RSA screening in couples and also literature suggests that paternal mutations probably lead to RSA [15]. The association between MTHFR C677T gene polymorphism with RSAs has been reported in women with RSA but with controversial results.
Although MTHFR C677T and A1298C mutations are observed in RSA, a meta-analysis report implies non significance with respect to MTHFR A1298C gene mutation [16]. Therefore, the present study focused to evaluate frequency of MTHFR C677T gene polymorphisms in couples with recurrent pregnancy loss and the impact of paternal polymorphisms of MTHFR C677T in recurrent pregnancy loss with RSA.
Materials and Methods
Patients: A total number of 30 couples were enrolled in the present study. Amongst, 15 couples (male n=15 and female n=15) with a history of two or more consecutive miscarriages with or without normal child termed as cases of RSA. Similarly 15 normal, healthy couples (male n=15 and female n=15) with minimum one or more normal live births without any history of abortions were included in the study. These patients were clinically diagnosed in Department of Obstetrics and Gynecology and were allowed to participate in the study after obtaining the patient consent form. After performing the karyotype for all the couples, those couples with chromosomal abnormality and uterine anomalies, genital infections, and endocrinological disorder were excluded from the present study.
Extraction of Genomic DNA: Blood samples were collected from couples belonging to the RSA group and the control group into sterile EDTA vaccutainer and were stored at 4°C until processing. Genomic DNA was isolated from the peripheral blood as per the standard procedure [15] that consists of erythrocyte lysis by Erythrocyte Lysis Buffer (ELB) till a white pellet free of heme was obtained. Leukocytes /lymphocytes lysis carried out by suspending white pellet in ELB and on mixing with 270μl of 20% SDS and 30μl of proteinase K (10mg/ml) were added and mixed. Samples were incubated at 370C, in water bath for overnight.
After 16 h of incubation, DNA was precipitated by isopropyl alcohol. The total precipitated DNA appeared as a white thread like structure was completely transferred to a sterile micro centrifuge tube containing 500μl of 80% alcohol and incubated at room temperature for 15 min. It was centrifuged at 12000 rpm for 5 min. The supernatant obtained was discarded and this step was repeated for three times to obtain purified form of DNA. The DNA was then air dried and dissolved in 500μl of Tris-EDTA buffer and then incubated at 65°C for 30 min. The dissolved fraction was refrigerated at 4°C for one day and stored at -20oC until use.
DNA Quantification by Agarose Gel Electrophoresis
The quantity of the DNA samples was checked in a 1% agarose gel. The solution was allowed to become lukewarm and 0.1mg/ml ethidium bromide was added. The gel was then poured on a gel-casting tray and allowed to solidify. The gel was placed in an electrophoresis tank with 1X TAE buffer. The DNA samples were mixed with 6X DNA loading dye and loaded on the gel. The gel was electrophoresed at 2 volts/cm and images were captured in a gel documentation system (Bio Rad Gel Doc) [17].
DNA Purity – Spectrophotometric Method
The quality and quantity of the DNA samples were assessed by spectrophotometric method by using Perkin Elmer Lambda 35 model. 50μl of TE buffer was pipetted into quartz cuvette and subjected for auto zero correction. 48μl of TE buffer and 2 μl of DNA sample were added in quartz cuvette, the absorbance was measured at 260 and 280nm. The absorbance at 260nm gives DNA concentration and the ratio between 260/280 gives the purity of DNA. DNA sample with 260/280 absorbance ratio between 1.7-1.9 were considered for PCR procedure. However, DNA samples with absorbance ratio less than 1.7 were subjected for precipitation until the desired absorbance was obtained. Then after, DNA sample of expected purity was used for PCR procedure.
Genotype Screening: For identification of MTHFR C677T gene mutation PCR-RFLP was performed in the present study.
PCR-RFLP –Based Screening: PCR amplification of the MTHFR C677T gene was amplified by polymerase chain reaction using primers as shown in [Table/Fig-1].
Sequence of primers, PCR product size, restriction enzymes and size of digested fragments that are used for screening by PCR-RFLP
| Mutation | Sequence of primers | PCR Product (bp) | Restriction enzyme | Wild type | Heterozygote | Mutant |
|---|
| MTHFR C677T | F-TGAAGGAGAAGGTGTCTGCGGGA | 198 | Hinf1 | 198 | 198/175/23 | 175/23 |
| R- AGGACGGTGCGGTGAGAGTC | | | | | |
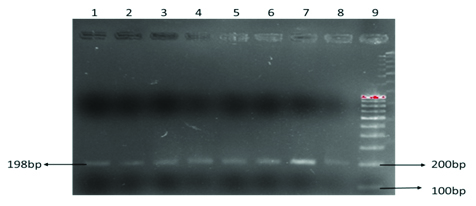
PCR was carried out on 50μl volume, in an Eppendorf thermal cycler and subjected to standardized PCR. Initial denaturation at 950C for 5 min followed by 35 cycles of denaturation at 950C for 1 min; annealing at 610C (MTHFR C677T) and extension at 720C for one minute and final extension at 720C for seven minutes were performed. PCR Amplification was confirmed by 2% agarose gel electrophoresis. 100bp DNA molecular weight marker was used to confirm the amplicon size. Electrophoresis was carried out at 80V for one hour and the gel was visualized in the gel documentation system as shown in [Table/Fig-2]. Finally, the amplified products were digested with 10U Hinf 1 restriction enzyme as shown in [Table/Fig-1] and digested products were separated on 2% agarose gel stained with Ethedium Bromide and visualized in the Gel Doc System.
Showing the PCR based amplified product of MTHFR gene (198bp)
Statistical Analysis
The percentage genotype distributions of each mutation, the frequency of heterozygous and homozygous were compared between the couples with RSA and healthy couples.
Results
The genotype distribution of each MTHFR mutation in RSA and healthy couples is shown in [Table/Fig-3,4,5,6,7,8,9,10]. Accordingly, the frequency of C677T genotype MTHFR gene in RSA Couples showed homozygous wild type CC (80% men & 86.6% women), heterozygous CT type (13.3% men & 13.3% women) and homozygous mutation TT type (6.67% men & 0% women). There was no difference observed in healthy couples with homozygous wild type CC 86.6%, heterozygous CT type 13.3%, and homozygous mutation TT type 0%. These results clearly indicate the decreased maternal genome risk of RSA in control groups showing no homozygous mutations of TT form.
Showing MTHFR C677T Genotypes
| Genotypes of MTHFR C677T | Couples with RSA (n=15) | Healthy Couples (n=15) |
|---|
| Males | Females | Males | Females |
|---|
| Homozygous Wild CC | 80% (12) | 86.6% (13) | 86.6% (13) | 86.6% (13) |
| Heterozygous CT | 13.3% (2) | 13.3% (2) | 13.3%(2) | 13.3% (2) |
| Homozygous Rare TT | 6.67% (1) | Nil | Nil | Nil |
Gender wise allele frequency in control and patient group
| Group | C allele | T allele |
|---|
| Control female (n= 15) | 0.93 | 0.07 |
| Control male (n= 15) | 0.93 | 0.07 |
| RSA female (n= 15) | 0.93 | 0.07 |
| RSA male (n= 15) | 0.87 | 0.13 |
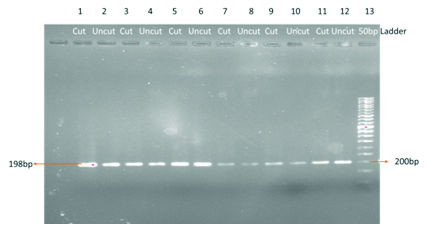
Showing homozygous CC genotype in control subjects
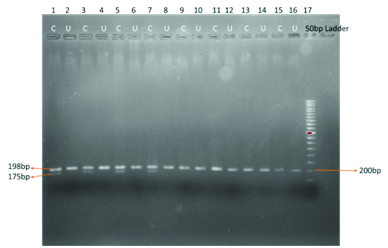
Showing heterozygous CT genotype in control subjects
Discussion
Despite a controversy reported on C677T gene polymorphism and miscarriage [16]. Few studies supported the direct evidence between C677T gene mutation and RSA across the globe [18–20]. Similarly, our study results also support the genetic analysis of MTHFR C677T gene polymorphism and clinically correlated with increased risk of miscarriage.
In one RSA couple, we observed a male patient with a homozygous TT mutation and a female with heterozygous CT mutation. The obstetric history reveals that they had four spontaneous abortions and a congenital anomalous baby. Since the MTHFR enzyme deficiency is autosomal recessive type, when both parents are carrying mutant allele the chance of inheritance of the offspring is much higher which may increase the risk of abortion by increasing the homocysteine levels in the mother as well as in the fetus and thereby increasing the thrombotic activity at the feto-placental interphase. Genetic analysis of this case is shown in the agarose gel electrophoretic pattern in [Table/Fig-7]. Line one showing the homozygous TT mutation in the affected male partner of the affected couple which is in agreement with a report of a study [21]. Line 3 showing the heterozygous CT mutation in female partner of affected couple.
RFLP analysis after digestion with Hin f 1 enzyme showing heterozygous C/T (female) & homozygous T/T (Male) mutant genotype in a couple. Line 1 showing homozygous T/T mutation with fragment length 175bp. Line 3 showing heterozygous C/T genotype with 198bp and 175bp length fragments. Line 5, 7, 9, 11 are homozygous C/C type with 198bp fragments. Line 2, 4, 6, 8, 10, 12 are uncut products to compare with the digested products. Line 13 is a 50bp DNA ladder
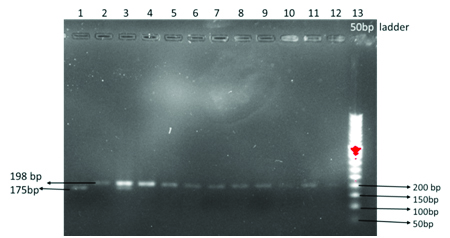
Ahmed et al., stated that woman with RSA has the C677T mutation identified by PCR – RFLP. Accordingly, our study also identified C677T mutation in RSA couple in comparison with control couples. In addition to this, in line one 198bp PCR product was chopped out by restriction enzyme into 175bp and 23bp fragments in both chromosomes. So198bp band cannot be seen in homozygous TT mutation. Only 175bp band and can be observed. In addition to homozygous TT mutation, in a male patient of RSA group we found another two males and one female patients carrying heterozygous mutant [22]. Genetic analyses of these cases are shown in [Table/Fig-8].
RFLP analysis after digestion with Hinf 1 showing heterozygous C/T, homozygous C/C genotype variants Line 1, 3, and 5 showing heterozygous C/T mutation. Line 7 is homozygous CC wild type which is normal. Lines 2,4,6,8 are undigested PCR products. Line 9 is a 100 bp DNA ladder
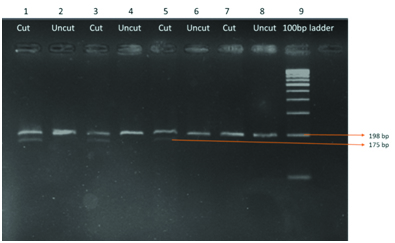
PCR product size is 198bp. After restriction digestion with Hinf 1 restriction enzyme for six hours at 370C, the mutant allele was digested into 175bp and 23bp fragments. In heterozygous (CT) two bands were observed with the base length of 198bp and 175bp. In case of normal homozygous (CC) only one band with 198bp length is observed. Allele frequency of MTHFR C677T gene in the population was calculated based on Hardy-Weinberg equilibrium. Allele frequency for T allele f (T) = CT + 2 X TT / 2 X CC + 2 X CT + 2 X TT: Allele frequency for C allele f (C) = CT+ 2 X CC / 2 X CC + 2 XCT + 2 X TT.
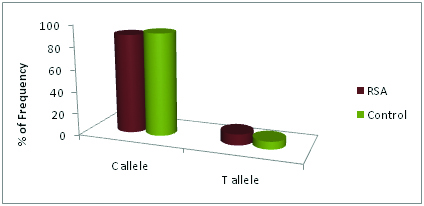
Increased frequency of mutant allele was observed in RSA couples when compared to healthy fertile couples. Mutant T allele frequency in the control group is 7%, whereas in RSA group it is 10%. The frequency of the C allele in control was observed as 93% and in the RSA group as 90% [Table/Fig-4,9,10]. This data shows that the frequency of occurrence of the mutant allele is much more in male partners of RSA cases. The distribution of allele frequency in normal women and women with RSA was identical.
the allele frequency in control and RSA groups
Gender wise Allele frequency in control and patient groups
Conclusion
Our research findings showed no significant variations in the MTHFR C677T genotype distribution among women who suffered from RSA and in controls. We also observed T allele frequency higher in the RSA group compared to control group and found variations in the MTHFR C677T genotype distribution among men who suffered from RSA impact. In one couple, we found a heterozygous mutation in female partner and homozygous mutation in the male partner. Increased frequency of T allele is also observed in paternal side in RSA couple. This study concludes that paternal screening is also essential along with maternal screening. However, there is a necessity to carry out similar studies with large sample size.
[1]. Hoff Man, Schorge Schaffer Halvorson Bradshaw Cunningham “First-Trimester Abortion”Williams Gynecology 2012 2nd edMcGraw-Hill Medical:170-79. [Google Scholar]
[2]. Mangalgiri Ashutosh S, Pathak SA, Cytogenetics in Recurrent AbortionsPeople’s Journal of Scientific Research 2008 1:5-8. [Google Scholar]
[3]. Olga BA Van den Akker, The psychological and social consequencies of miscarriageExpert review. Obstet. Gynecol 2011 6(3):295-04. [Google Scholar]
[4]. Yumi Nakano, Tatsuo Akechi, Mayumi Sugiura Ogasawara, Cognitive behavior therapy for psychological distress in patients with recurrent miscarriagePsychology Research and Behavior Management 2013 6:37-43. [Google Scholar]
[5]. Simpson J, Carson S, Glob. librwomen's med 2013 DOI 10.3843/GLOWM.10319 [Google Scholar]
[6]. Arjun G, Recurrent early pregnancy lossAn Introduction to Genetics and Fetal Medicine 2000 :27-30. [Google Scholar]
[7]. Chaithra PT, Suttur S, Malini C, Sharath Kumar, An Overview of Genetic and Molecular Factors Responsible for Recurrent Pregnancy LossInt J Hum Genet 2011 11(4):217-25. [Google Scholar]
[8]. Fryns JP, Van Buggenhout G, Structural chromosome rearrangements in couples with recurrent fetal wastageEur J ObstetGynecolReprod Biol 1998 81:171-76. [Google Scholar]
[9]. Forges T, Monnier-Barbarino P, Alberto JM RM, Impact of folate and homocysteine metabolism on human reproductive healthHuman reproductive update 2007 13(3):225-38. [Google Scholar]
[10]. Anne-MarieNybo Andersen, Jan Wohlfahrt, Peter Christens, Jorn Olsen, Mads Melbye, Maternal age and fetal loss: population based register linkage studyBMJ 2000 320:1708-12. [Google Scholar]
[11]. Chaithra PT, Suttur S, Malini C, Sharath Kumar, An Overview of Genetic and Molecular Factors Responsible for Recurrent Pregnancy LossInt J Hum Genet 2011 11(4):217-25. [Google Scholar]
[12]. Ivy Altomare, Alan Adler, Louis M Aledort, The 5, 10 methylenetetrahydrofolate reductase C677T mutation and risk of fetal loss: a case series and review of the literatureThrombosis Journal 2007 5:17doi:10.1186/1477-9560-5-17 [Google Scholar]
[13]. Ray JC, Laskin CA, Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption , pre- eclampsia and spontaneous pregnancy loss : a systemic reviewPlacenta 1999 20:519-29. [Google Scholar]
[14]. Devi A Radha Rama, Govindaiah V, Ramakrishna G, Maushad SM, Prevalence of methylene tetrahydrofolate reductase polymorphism in south Indian populationCurrent science 2004 86(3):440-43. [Google Scholar]
[15]. Jivraj S, Rai R, Underwood J, Regan L, Genetic thrombophilic mutations among couples with recurrent miscarriageHuman Reproduction 2006 21(5):1161-65. [Google Scholar]
[16]. Cao Y, Xu J, Zhang Z, Association study between methylene tetrahydrofolate reductase polymorphism and unexplained recurrent pregnancy loss- A meta-analysisGene 2013 514(2):105-11. [Google Scholar]
[17]. Rousseau F, Rehel R, Rouillard P, DeGranpre P, Khandjian EW, High throughput and economical mutation detection and RFLP analysis using a minimethod for DNA preparation from whole blood and acrylamide gel electrophoresisHuman Mutation 1994 4(1):51-54. [Google Scholar]
[18]. Morales-Machin A, Borjas-Fajardo L, Quintero JM, C677T polymorphism of the methylentetrahydrofolate reductase gene as risk factor in women with recurrent abortionInvest Clin 2009 50(3):327-33. [Google Scholar]
[19]. Mukhopadhyay R, Saraswathy KN, Ghosh PK, MTHFR C677T and factor V Leiden in recurrent pregnancy loss: a study among an endogamous group in North IndiaGenet Test Mol Biomarkers 2009 13(6):861-65. [Google Scholar]
[20]. Pickell L, Li D, Brown K, Mikael LG, Wang XL, Wu Q, Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in miceBirth Defects Res A ClinMolTeratol 2009 85(6):531-41. [Google Scholar]
[21]. Rodríguez-GuillénMdel R, Maternal MTHFR polymorphisms and risk of spontaneous abortionSaludPublica Mex 2009 51(1):19-25. [Google Scholar]
[22]. Govindaiah V, Naushad SM, Prabhakara K, Krishna PC, Devi Radha Rama A, Association of parental hyperhomocysteinemia and C677T Methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy lossClinBiochem 2009 42(4-5):380-86. [Google Scholar]