Efficacy of Garcinia Cambogia on Body Weight, Inflammation and Glucose Tolerance in High Fat Fed Male Wistar Rats
Ramalingam Sripradha1, Sridhar Gopalakrishna Magadi2
1 Scholar, Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherr, India.
2 Senior Professor, Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sridhar Gopalakrishna Magadi, Senior Professor, Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry – 605006, India. E-mail : sridhar_biochem@yahoo.co.in
Introduction: Obesity leads to derangements in lipid and glucose homeostasis resulting in various metabolic complications. Plants containing vital phytochemicals are known to posses anti obesity properties and have proved to exert beneficial effects in obesity.
Objectives: The present study was aimed to investigate the effects of Garcinia Cambogia on body weight, glucose tolerance and inflammation in high fat diet fed male Wistar rats.
Materials and Methods: Five month old male wistar rats (n=40) were divided into four groups. Two groups were fed with standard rodent diet and the remaining two with 30% high fat diet. One group in each of the two sets received the crude ethanolic extract of Garcinia Cambogia at a dose of 400mg/kg body weight/day for ten weeks. Body weight, intraperitoneal glucose tolerance test, leptin, tumour necrosis factor-α (TNF-α) and renal function (urea, creatinine, uric acid) were studied.
Results: High fat diet fed rats showed increased body weight gain, glucose intolerance, elevated levels of plasma leptin and TNF-α. Supplementation of Garcinia Cambogia extract (GE) along with high fat diet significantly decreased body weight gain, glucose intolerance, plasma leptin and TNF-α level. No significant changes were observed in the renal function parameters in any of the groups.
Conclusion: Supplementation of the Garcinia Cambogia extract with high fat diet reduced body weight gain, inflammation and glucose intolerance.
Hydroxycitric acid, Leptin, Obesity, TNF- α
Introduction
Obesity occurs due to imbalance between food consumption and energy expenditure [1]. It is a vital risk factor for the global burden of chronic disease and disability [2]. Obesity increases the risk of several complications like diabetes mellitus, cardiovascular disease, hypertension and certain cancers [3]. Chronic consumption of fat rich diet leads to dyslipidemia which is characterized by increased plasma levels of total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C) and decreased high density lipoprotein cholesterol (HDL-C) [4,5]. Excess fat intake leads to accumulation of lipid in the adipocytes causing hypertrophy of the adipose tissue which produces inflammatory cytokines like TNF-α, resistin, interleukin -6 (IL-6), plasminogen activator inhibitor-1 (PAI-1) and monocyte chemoattractant protein -1 (MCP-1). These cytokines cause local and systemic insulin resistance leading to impairment in insulin signaling [6].The elevated cytokines also contribute for the development of renal pathologies in diet induced obese animal models [7]. Though several pharmacological modalities are available in the treatment of obesity and its associated complications, there is considerable interest in the naturally available plant products with antiobesity properties.
Phytochemicals possessing antiobesity effects with minimal side effects would be beneficial in the management of obesity. Garcinia Cambogia (Garcinia gummi-gutta) is a tropical edible fruit of the family Clusiaceae grown in south East Asia, south India and Africa. It has a characteristic sweet and sour taste, used commonly for preparing culinary dishes [8]. The fruit rinds of G. cambogia contain 20-30% of (-) Hydroxycitric acid (HCA) [9] which is a competitive inhibitor of the enzyme ATP-citrate lyase, a key enzyme involved in fatty acid and cholesterol biosynthesis (EC 4.1.3.8) [10]. The inhibitory effect of HCA on ATP citrate lyase lowers the production of cholesterol, fatty acids and also promotes gluconeogenesis and glycogenesis [11]. Apart from HCA, the fruits of G. cambogia have also been reported to contain secondary metabolites like xanthones [12], flavonoids [13], benzophenones like Garcinol/camboginol and Isoxanthohumol [14]. All the three constituents have been reported to possess antioxidant properties. Several animal and human studies have been carried out with HCA, only a few evidences are available in the literature about the beneficial effects of the crude extract of Garcinia Cambogia. Thus the present study was designed to investigate the effects of Garcinia Cambogia on body weight, glucose tolerance and inflammation in rats fed with high fat diet.
Materials and Methods
Preparation of crudeGarcinia Cambogia extracts (GE): The Garcinia Cambogia fruits were obtained from Kerala. The fruits were washed, deseeded and the rinds were sun dried. The fruit rinds were crushed, ground and extracted crudely with 70% v/v ethanol with shaking at room temperature for 24 h. The solution was filtered and the filtrate was evaporated under reduced pressure. The semi solid residual material was collected and stored at 40C till use [15].
Animals and study design
The present study was approved by the Institute Scientific Advisory Committee and Animal Ethics Committee. Five month old male Wistar rats weighing 200 to 250 gm were housed in polypropylene cages with stainless steel grill. The animals were maintained at standard conditions with a 12:12 h light: dark cycle. After acclimatization the rats were randomly divided into four groups (10 rats / group) based on their body weight.
Group 1: Control+ vehicle, the rats received standard rodent diet.
Group 2: Control + GE, the rats received standard rodent diet along with GE.
Group 3: High fat diet +vehicle, rats received 30% High fat diet (HFD).
Group 4: High fat diet +GE, rats received 30% High fat diet (HFD) along with GE
The G. cambogia extract was dissolved in drinking water and administered at a dose of 400mg/Kg body wt/day/rat for an experimental duration of ten weeks [15]. Drinking water was given as vehicle for control and HFD groups.
Composition of High fat diet
| Diet Ingredients | (g/100g) |
|---|
| • | Casein | 26 |
| • | Corn starch | 16 |
| • | Sucrose | 16 |
| • | Cellulose | 6.1 |
| • | Safflower oil | 1 |
| • | Butter | 29 |
| • | Standard Mineral mixture | 4.2 |
| • | Standard Vitamin mixture | 1.2 |
| • | Choline | 0.2 |
| • | DL-Methionine | 0.3 |
The non-purified high-fat diet was prepared as described by Hsu et al., [16], with 53.78% of total calories derived from fat, 21.8% from protein, and 30.35 % from carbohydrate. The energy of the high-fat diet was 5.02 kcal/g.
Sample collection
At the end of the experiment fasting blood samples were collected and centrifuged at 3500 rpm. The plasma was separated, aliquoted and stored at -800C for further analysis.
Intra peritoneal glucose tolerance test (IPGTT)
Intraperitoneal glucose tolerance test was performed for all rats at the end of the experiment. After 12-15 h of overnight fasting, basal blood samples were collected. Glucose (2g/Kg BW in saline) was administered intraperitoneally and blood samples were collected at 30, 60 and 120 min interval [17]. The plasma was separated and glucose levels were estimated immediately.
Biochemical analyses
Plasma glucose, urea, creatinine and uric acid were estimated using standard reagent kits adapted to a fully automated clinical chemistry analyser (AU-400, OLYMPUS, UK). The plasma leptin levels were estimated using Ray BioR rat leptin ELISA kit (Ray Biotech, Inc. USA) and TNF- α levels using Diaclone rat TNF- α ELISA Kit (Diaclone, France).
Statistical Analysis
Results were expressed as Mean ± SD. One-way analysis of variance (ANOVA) with Tukey as post hoc test was used to evaluate the differences between the groups. Statistical Package of Social Service (SPSS version 19) was used for performing statistical analyses. Area under curve (AUC) was analysed using Graph pad Prism 6 software. P-value <0.05 was considered as statistically significant.
Results
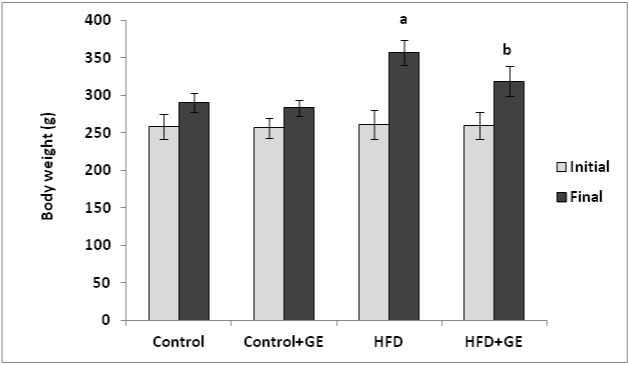
[Table/Fig-1] show rats fed with high fat diet showed significant increase in body weight gain (p<0.001) when compared to control rats. Supplementation with GE along with HFD showed significant decrease in the body weight gain (p<0.001).
Effect of GE on body weight in control and high fat fed rats. All values were expressed as Mean ± SD. N=10 rats/ group. a,b P< 0.05 was considered as statistically significant. a vs Control group. b vs HFD group
[Table/Fig-2] show high fat diet fed rats showed elevated plasma levels of leptin (p<0.001) and TNF-α (p<0.001) when compared to control rats. GE treatment along with HFD decreased leptin (p=0.031) and TNF-α (p=0.013) levels significantly. No significant changes were observed with the renal function parameters urea, creatinine and uric acid in any of the groups.
Effect of GE on plasma leptin, TNF-α, urea, creatinine and uric acid in control and high fat fed rats. All values were expressed as Mean ± SD. N=10 rats/ group. a,b P< 0.05 was considered as statistically significant. a vs Control group. b vs HFD group
| Sl.No | Parameters | Control | Control+GE | HFD | HFD+GE |
|---|
| 1 | Leptin (ng/ml) | 0.94±0.25 | 0.92±0.20 | 1.95±0.57a | 1.47±0.35b |
| 2. | TNF-α (pg/ml) | 18.53±4.86 | 16.20±3.82 | 48.30±8.97a | 39.80±4.28b |
| 3. | Urea (mg/dl) | 31.40±4.43 | 30.40±2.46 | 27.20±3.65 | 28.20±4.52 |
| 4. | Creatinine (mg/dl) | 0.64±0.05 | 0.63±0.06 | 0.60±0.07 | 0.61±0.03 |
| 5. | Uric acid (mg/dl) | 0.63±0.08 | 0.62±0.09 | 0.58±0.08 | 0.59±0.09 |
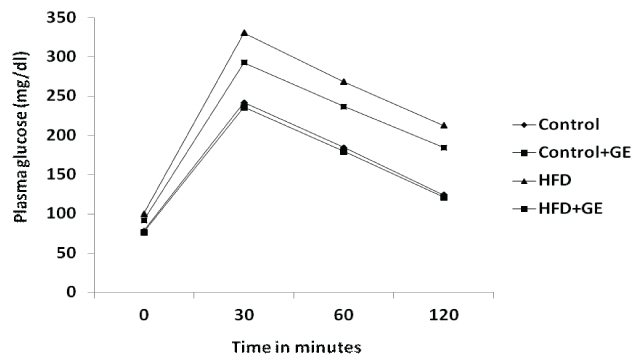
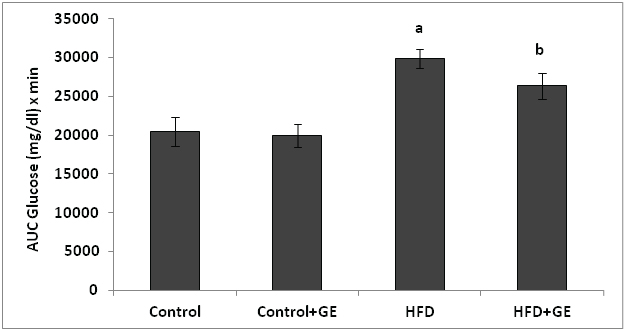
[Table/Fig-3a,b] shows that there was significant increase in the IPGTT and incremental area under curve (p<0.001) of IPGTT in rats fed with high fat diet when compared to control rats. GE administration along with HFD showed significant decrease in the IPGTT and incremental area under curve (p<0.001).
Effect of GE on intraperitoneal glucose tolerance test (IPGTT) in control and high fat fed rats
All values were expressed as Mean ± SD. N=10 rats/ group. a,b P< 0.05 was considered as statistically significant. a vs Control group. b vs HFD group
Effect of GE on incremental area under curve (AUC) of IPGTT in control and high fat fed rats
All values were expressed as Mean ± SD. N=10 rats/ group. a,b P< 0.05 was considered as statistically significant. a vs Control group. b vs HFD group
Discussion
The persistent rise in the incidence of obesity and its associated complications continues to present monumental challenges to health. Ingestion of excess calories has become a major risk factor for the occurrence of obesity [18]. Leptin, the adipose derived hormone regulates food intake and energy expenditure through interactions with hypothalamic nuclei. Hence it plays a crucial role in the maintenance of body weight [19]. The circulating leptin level is proportional to body fat but in case of diet induced obesity the increased leptin level fails to prevent weight gain. Such unresponsiveness of endogenous or exogenous leptin is referred as ‘leptin resistance’ [20]. In our study we found increased body weight gain and plasma leptin level in rats fed with high fat diet. This finding was consistent with the previous literature [21]. Supplementation of GE along with high fat diet showed decreased gain in body weight and plasma leptin level. GE contains hydroxycitric acid (HCA) which is a competitive inhibitor of the citrate cleavage enzyme, ATP citrate lyase [21]. Due to concentration of the GE, the free form of HCA is converted to its lactone form (HCAL). The HCAL is relatively stable but less effective when compared to the free HCA [22]. Previous reports have shown that HCAL is effective in reducing food intake and body weight [23]. The decrease in body weight and leptin observed in our study might be due to the inhibitory effect of HCAL on calorie intake.
Lipid laden adipocytes show increase in the production of proinflammatory cytokines TNF-α, IL-6, resistin, MCP-1 and PAI-1. These cytokines up regulate the synthesis of adhesion molecules (intracellular adhesion molecule and vascular cell adhesion molecule). Cross talks between endothelial cells, adipocytes and macrophages enhance the inflammatory state leading to synthesis of excess cytokines. All these consequences culminate in systemic and local insulin resistance causing impairment in insulin sensitivity [6]. Evidences suggest that in cultured human muscle cells TNF-α affects insulin stimulated storage of glucose and impairs insulin signaling [24]. Also, it was reported that TNF-α knocked out obese mice showed protection against insulin resistance [25].These evidences provide the direct role of TNF-α in insulin resistance. To assess the insulin sensitivity generally glucose tolerance test is performed. Rats fed with fat rich diet showed elevated levels of TNF-α and glucose intolerance. Our results are in agreement with the previous reports [26,27]. Treatment with GE significantly decreased plasma levels of TNF- α and improved glucose intolerance. In addition to HCAL, the GE also contains xanthones [12], flavonoids [13] and benzophenones [14] which are known to have antioxidant properties. The polyisoprenylated benzophenone garcinol/ camboginol and isoxanthohumol are reported to be present in the fruit rinds of Garcinia Cambogia [22]. The garcinol has been shown to posses antioxidative, anti-inflammatory and antiproliferative properties [28]. In vitro assays have also reported the antioxidant properties of Garcinia Cambogia [29]. A study in catfish have stated that administration of the crude aqueous extract of Garcinia Cambogia fruit reduced body weight gain, glucose, TC, TG, LDL and increased red blood cells (RBC), white blood cells (WBC), platelets, HDL [30]. The antioxidants present in the Garcinia Cambogia fruit might have contributed for the significant reduction in the TNF-α level and glucose intolerance.
In diet induced obese rats the elevated levels of proinflammatory cytokines cause monocyte infiltration and leads to renal damage [7]. To assess the renal function plasma levels of urea, creatinine, and uric acid were estimated. There was no statistical significance in all parameters in any of the groups. There was mild decrease in the levels of blood urea, creatinine and uric acid in high fat rats when compared to control rats. The low protein content in the high fat diet than the standard rodent diet might be the reason for the observed decrease in the levels of urea, creatinine and uric acid.
Conclusion
Supplementation of Garcinia Cambogia showed decrease in body weight gain, inflammation and glucose intolerance. This supports the popular view that inclusion of Garcinia Cambogia in the diet may help in body weight management. However scientific studies to confirm these effects in humans are warranted.
[1]. Wang C-Y, Liao JK, A mouse model of diet-induced obesity and insulin resistanceMethods Mol Biol Clifton NJ 2012 821:421-33. [Google Scholar]
[2]. Fujioka K, Management of obesity as a chronic disease: nonpharmacologic, pharmacologic, and surgical optionsObes Res 2002 10(Suppl 2):116S-23S. [Google Scholar]
[3]. Unger RH, Lipid overload and overflow: metabolic trauma and the metabolic syndromeTrends Endocrinol Metab TEM 2003 14(9):398-403. [Google Scholar]
[4]. Kainuma M, Fujimoto M, Sekiya N, Tsuneyama K, Cheng C, Takano Y, Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosisJ Gastroenterol 2006 41(10):971-80. [Google Scholar]
[5]. Sikder K, Das N, Kesh SB, Dey S, Quercetin and beta-sitosterol prevent high fat diet induced dyslipidemia and hepatotoxicity in Swiss albino miceIndian J Exp Biol 2014 52(1):60-66. [Google Scholar]
[6]. Shoelson SE, Herrero L, Naaz A, Obesity, inflammation, and insulin resistanceGastroenterology 2007 132(6):2169-80. [Google Scholar]
[7]. Stemmer K, High-fat-diet-induced obesity causes an inflammatory and tumour-promoting microenvironment in the rat kidneyDis Model Mech 2012 5(5):627-35. [Google Scholar]
[8]. Ohia SE, Opere CA, LeDay AM, Bagchi M, Bagchi D, Stohs SJ, Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX)Mol Cell Biochem 2002 238(1-2):89-103. [Google Scholar]
[9]. Lewis YS, Neelakantan S, (-)-Hydroxycitric acidsThe principal acid in the fruits of Garcinia CambogiaPhytochemistry 1965 4:619-25. [Google Scholar]
[10]. Sullivan AC, Singh M, Srere PA, Glusker JP, Reactivity and inhibitor potential of hydroxycitrate isomers with citrate synthase, citrate lyase, and ATP citrate lyaseJ Biol Chem 1977 252(21):7583-90. [Google Scholar]
[11]. Jena BS, Jayaprakasha GK, Singh RP, Sakariah KK, Chemistry and biochemistry of (-)-hydroxycitric acid from GarciniaJ Agric Food Chem 2002 50(1):10-22. [Google Scholar]
[12]. Iinuma M, Ito T, Miyake R, Tanaka T, Chelladurai V, A xanthone from Garcinia CambogiaPhytochemistry 1998 47(6):1169-70. [Google Scholar]
[13]. Koshy AS, Anila L, Vijayalakshmi NR, Flavonoids from Garcinia Cambogia lower lipid levels in hypercholesteromic ratsFood chemistry 2001 72(3):289-94. [Google Scholar]
[14]. Masullo M, Bassarello C, Suzuki H, Pizza C, Piacente S, Polyisoprenylated benzophenones and an unusual polyisoprenylated tetracyclic xanthone from the fruits of Garcinia CambogiaJ Agric Food Chem 2008 56(13):5205-10. [Google Scholar]
[15]. Oluyemi KA, Omotuyi IO, Jimoh OR, Adesanya OA, Saalu CL, Josiah SJ, Erythropoietic and anti-obesity effects of Garcinia Cambogia (bitter kola) in Wistar ratsBiotechnol Appl Biochem 2007 46(Pt 1):69-72. [Google Scholar]
[16]. Hsu S-C, Huang C-J, Reduced fat mass in rats fed a high oleic acid-rich safflower oil diet is associated with changes in expression of hepatic PPARalpha and adipose SREBP-1c-regulated genesJ Nutr 2006 136(7):1779-85. [Google Scholar]
[17]. Huang B-W, Chiang M-T, Yao H-T, Chiang W, The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in ratsDiabetes Obes Metab 2004 6(2):120-26. [Google Scholar]
[18]. Wisse BE, Kim F, Schwartz MW, Physiology. An integrative view of obesityScience 2007 318(5852):928-29. [Google Scholar]
[19]. Friedman JM, Halaas JL, Leptin and the regulation of body weight in mammalsNature 1998 395(6704):763-70. [Google Scholar]
[20]. Scarpace PJ, Zhang Y, Elevated leptin: consequence or cause of obesity?Front Biosci 2007 12:3531-44. [Google Scholar]
[21]. Kim K-Y, Lee HN, Kim YJ, Park T, Garcinia Cambogia extract ameliorates visceral adiposity in C57BL/6J mice fed on a high-fat dietBiosci Biotechnol Biochem 2008 72(7):1772-80. [Google Scholar]
[22]. Chuah LO, Ho WY, Beh BK, Yeap SK, Updates on Antiobesity Effect of Garcinia Origin (-)-HCAEvid-Based Complement Altern Med ECAM 2013 2013:751658 [Google Scholar]
[23]. Venkateswara G Rao, Karunakara AC, Babu RR Santhosh, Ranjit D, Reddy G Chandrasekara, Hydroxycitric acid lactone and its salts: Preparation and appetite suppression studiesFood Chemistry 2010 120:235-39. [Google Scholar]
[24]. Halse R, Pearson SL, McCormack JG, Yeaman SJ, Taylor R, Effects of tumour necrosis factor-alpha on insulin action in cultured human muscle cellsDiabetes 2001 50(5):1102-09. [Google Scholar]
[25]. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS, Protection from obesity-induced insulin resistance in mice lacking TNF-alpha functionNature 1997 389(6651):610-14. [Google Scholar]
[26]. Borst SE, Conover CF, High-fat diet induces increased tissue expression of TNF-alphaLife Sci 2005 77(17):2156-65. [Google Scholar]
[27]. Sridhar MG, Vinayagamoorthi R, Arul Suyambunathan V, Bobby Z, Selvaraj N, Bitter gourd (Momordica charantia) improves insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in high-fat-fed ratsBr J Nutr 2008 99(4):806-12. [Google Scholar]
[28]. Saadat N, Gupta SV, Potential role of garcinol as an anticancer agentJ Oncol 2012 2012:647206 [Google Scholar]
[29]. Subhashini N, Nagarajan G, Kavimani S, In vitro antioxidant and anticholinesterase activities of garcinia combogiaInt J Pharm Pharm Sci 2011 3(3):129-32. [Google Scholar]
[30]. Prasad G, Priyanka GL, Effect of fruit rind extract of Garcinia gummi-gutta on haematology and plasma biochemistry of Catfish Pangasianodon hypophthalmusAsian Journal of Biochemistry 2011 6(3):240-51. [Google Scholar]