Adenosquamous Variant of Metaplastic Carcinoma of Breast – An Unusual Histological Variant
P.U. Swathy1, P. Arunalatha2, K. Chandramouleeswari3, S. Mary Lily4, S. Ramya5
1 Post Graduate, Department of Pathology, Stanley Medical College, Chennai, Tamilnadu, India.
2 Professor, Department of Pathology, Stanley Medical College, Chennai, Tamilnadu, India.
3 Professor, Department of Pathology, Stanley Medical College, Chennai, Tamilnadu, India.
4 Head of the Department, Department of Pathology, Stanley Medical College, Chennai, Tamilnadu, India.
5 Post Graduate, Department of Pathology, Stanley Medical College, Chennai, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. P.U.Swathy, No: 42/4 New Thandavaryan Street, Old Washermenpet, Chennai-21, India. E-mail : swathyumashankar@gmail.com
Metaplastic carcinoma of breast refers to a heterogeneous group of neoplasms characterized by intimate admixture of adenocarcinoma with dominant areas of spindle cell, squamous cell and/ or mesenchymal differentiation. They constitute the rarest histological variant of invasive ductal carcinoma. These carcinomas have aggressive clinical behaviour and show suboptimal response to standard treatment. A 49-year-old female presented with lump in the left breast for one year. She was diagnosed as infiltrating ductal carcinoma breast with triple negative hormone status by trucut biopsy. She completed four cycles of neoadjuvant chemotherapy. Postchemotherapy, axillary nodes decreased in size but the size of the primary tumour remained the same. Hence, she underwent modified radical mastectomy and the specimen sent for histopathological examination. Grossly, there was a solitary cyst measuring 4x3cm. Histologically, cyst enclosing malignant cells which resemble mature squamous epithelial cells. Also, seen are malignant cells in glandular pattern.
Chemotherapy, Cyst, Neoplasms, Squamous epithelial cells
Case Report
A 49-year-old female presented with breast lump for one year. On palpation, it was 4x3 cm hard lump with nipple retraction and axillary lymph node measuring 3x3 cm. Fine needle aspiration of the lump was reported as ductal carcinoma. Trucut biopsy confirmed the diagnosis of intraductal carcinoma of breast. This tumour was negative for estrogen, progesterone and HER2/neu receptors. She received four cycles of neoadjuvant chemotherapy as per the oncologist’s opinion. After the completion of the chemotherapy, axillary lymphnodes regressed but the primay tumour was unmodified. Hence, she underwent modified radical mastectomy and the specimen sent for histopathological examination.
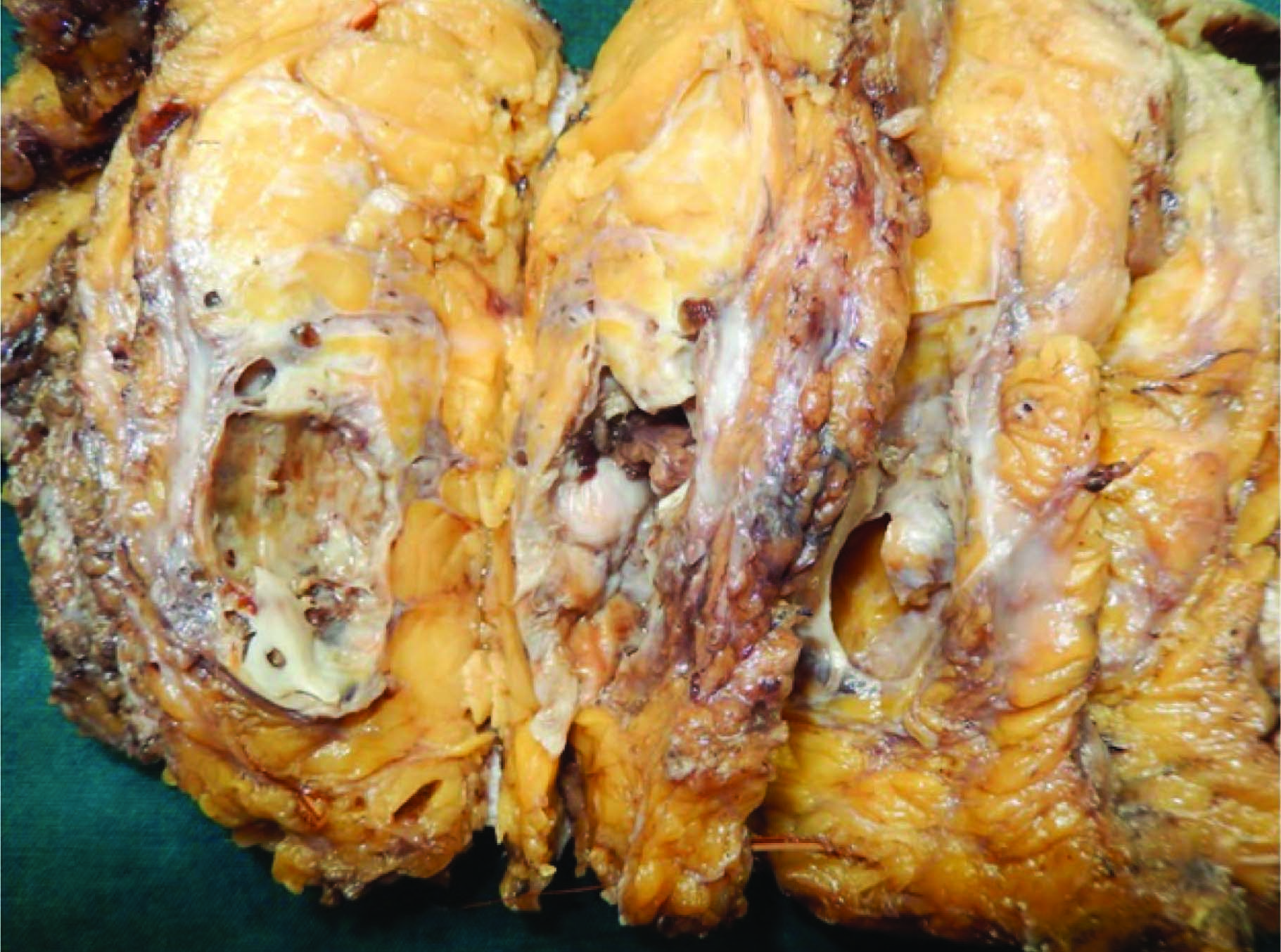
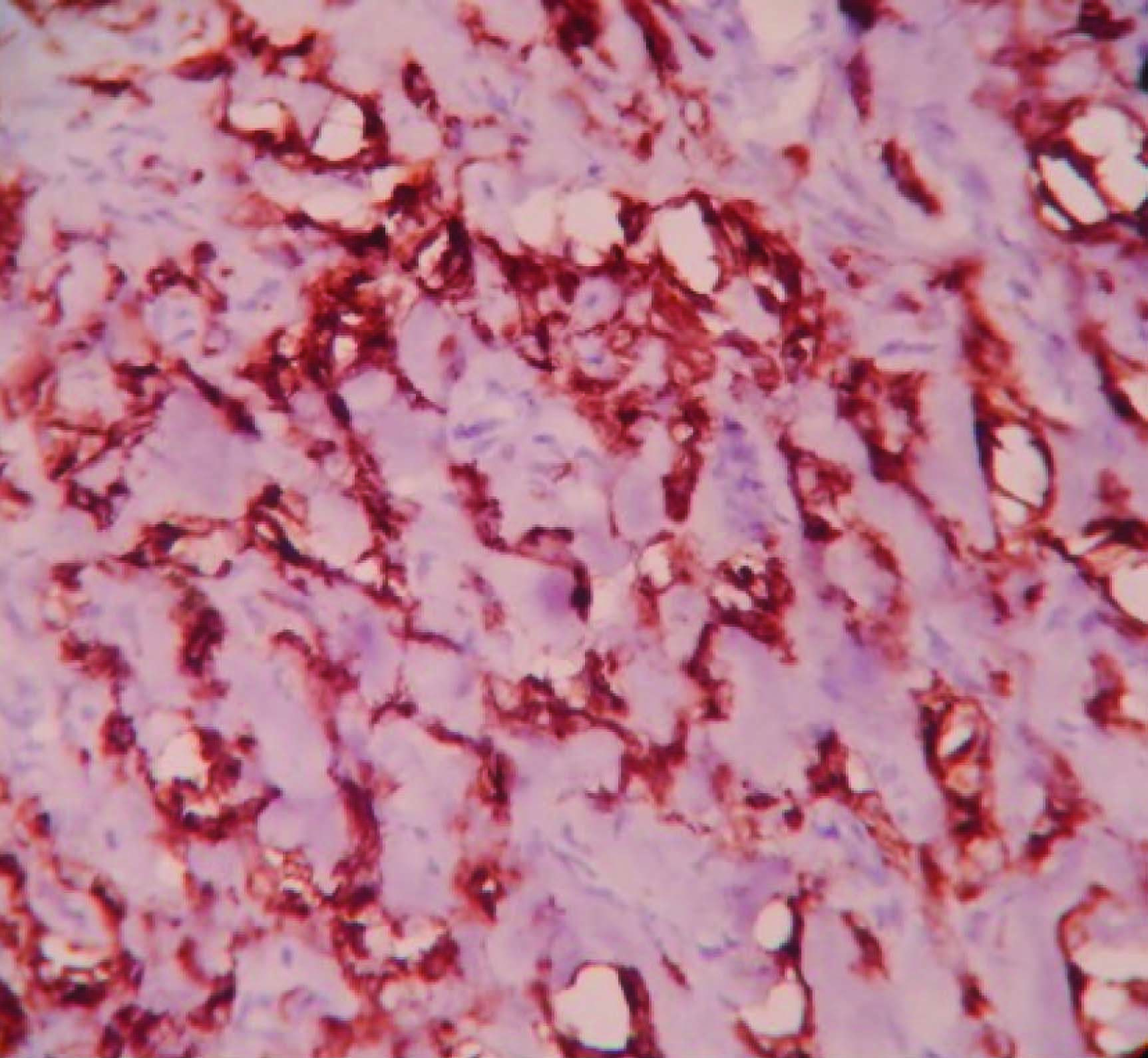
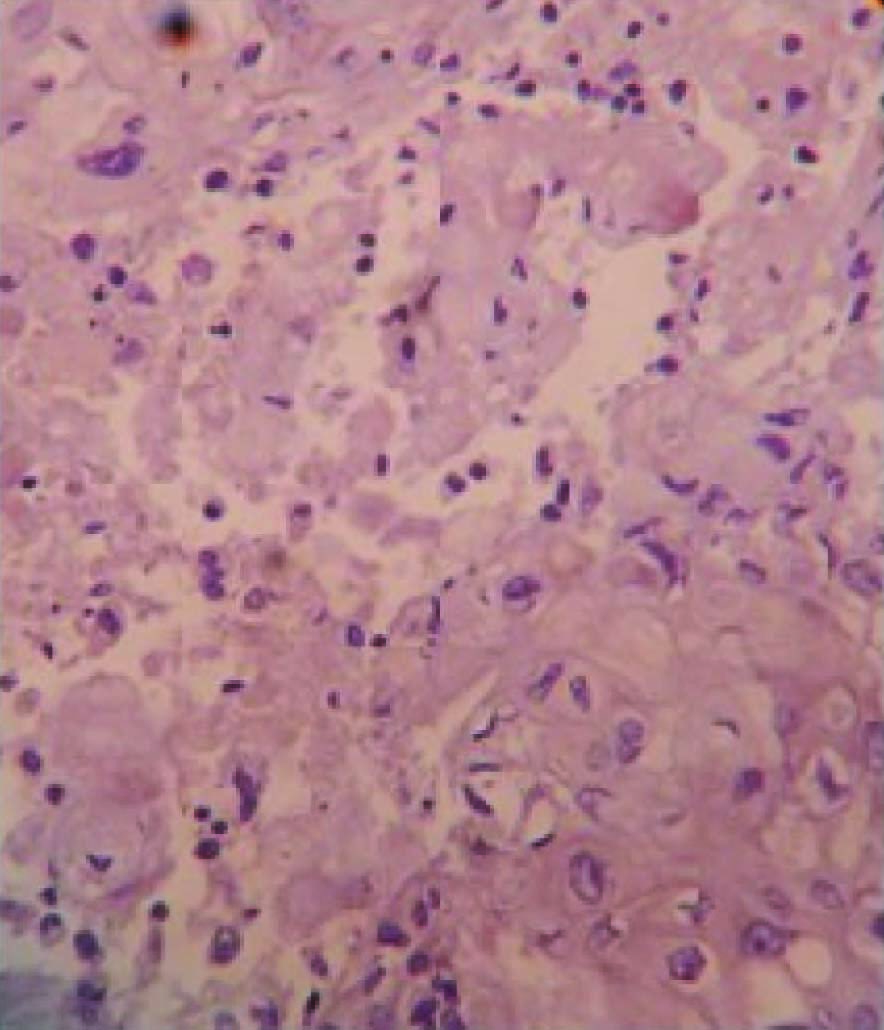
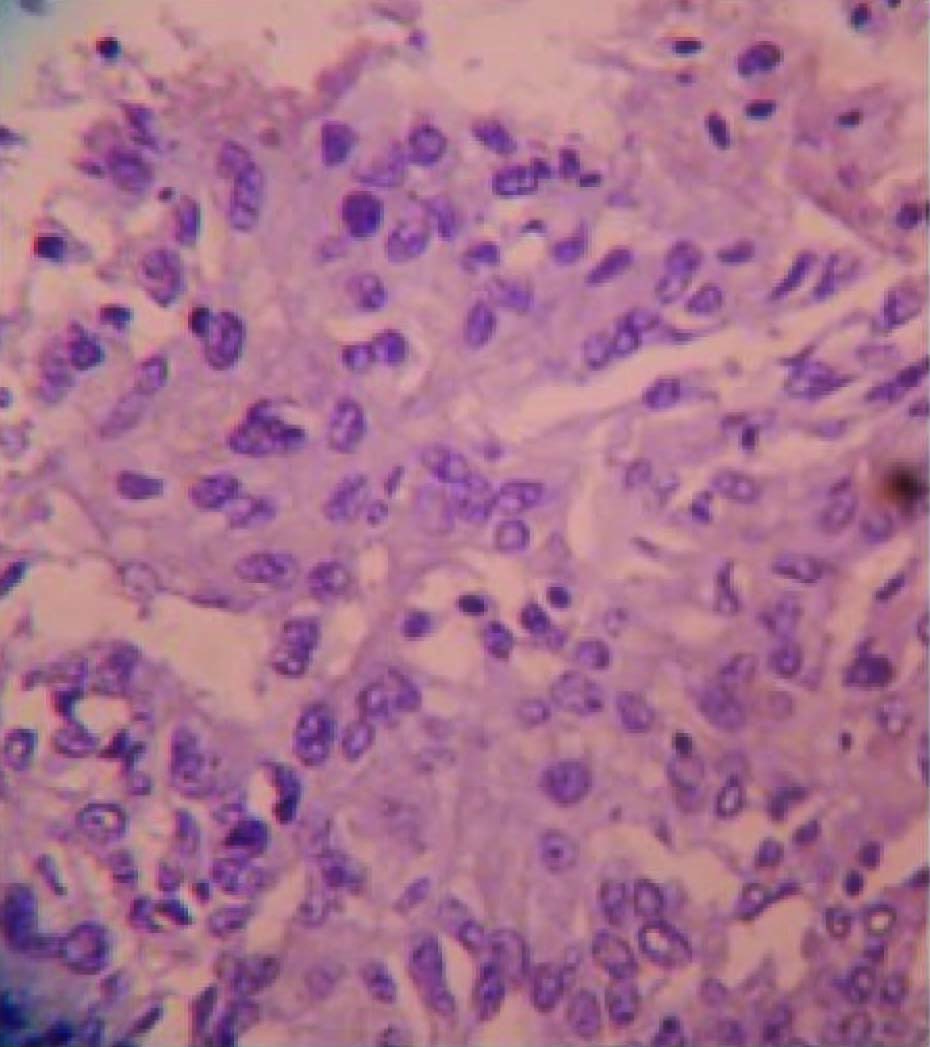
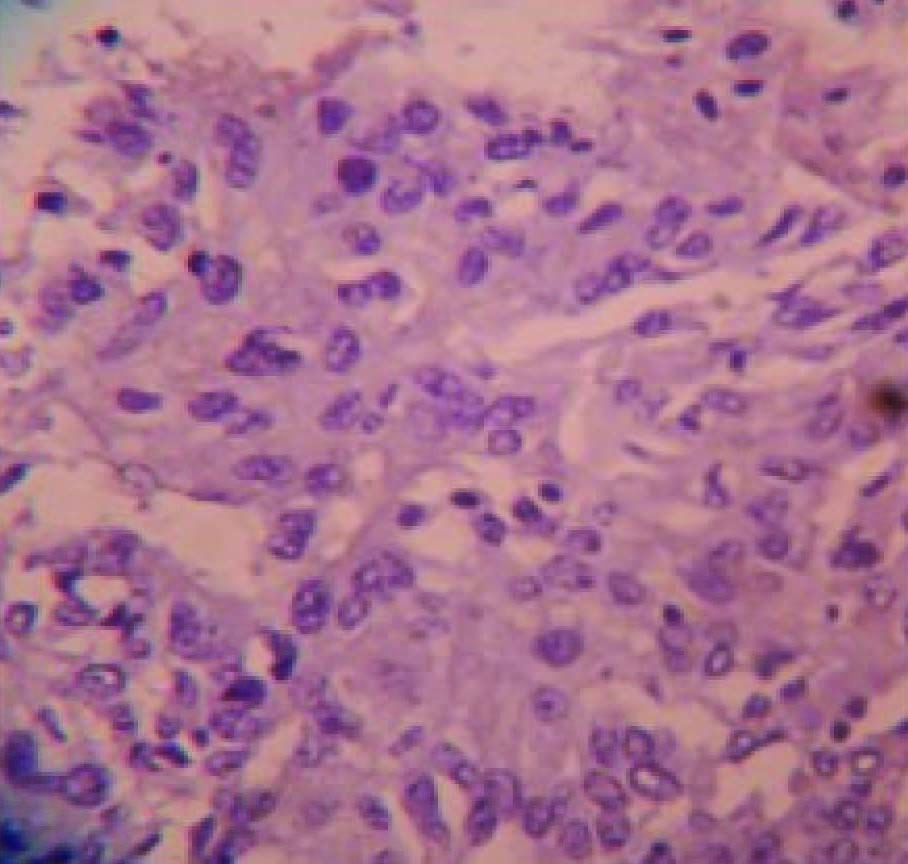
Grossly, modified radical mastectomy with elliptical piece of skin measuring 20x15x8cms. Multiple cut sections revealed solitary cyst enclosing focal pearly white solid areas measuring 4x3x3cm [Table/Fig-1]. All resected margins are free of tumour. Axillary pad of fat revealed seven nodes. Microscopy revealed a cyst enclosing solid nests of malignant cells which resemble mature squamous epithelial cells intimately admixed with gland formation [Table/Fig-2&3]. Immunohistochemistry for ER, PR, HER2 and Cytokeratin done [Table/Fig-4,5,6,7,8].
Gross – cystic lesion with focal solid pearly white areas
Malignant squamous epithelial cells

To the left- malignant squamous epithelial cells, To the right – glandular pattern of neoplastic cells

Comparison of immunonhistochemical markers before and after chemotherapy
| Type of Biopsy | ER,PR,HER2* | CK** |
|---|
| Trucut biopsy | Negative | Not done |
| Modified radical mastectomy | Negative | Strongly positive |
* ER- estrogen receptor, PR- progesterone receptor, HER2- Her2neu ,** CK- cytokeratin
Discussion
Adenosquamous carcinomas of the breast are rare tumours, included in the last edition of the World Health Organization (WHO) classification of breast cancers [1,2] as a subtype of metaplastic carcinoma. They are characterized by well-developed gland/tubule formation intimately admixed with solid nests of squamous cells in a spindle cell background. It constitute 0.3% of all breast cancers. It was first described in early 1980’s [3,4]. Adenosquamous carcinomas are subdivided into low grade and high grade. Low grade adenosquamous do not have obvious nuclear anaplasia, do not metastatise and overall good prognosis. In contrast, High grade adenosquamous are quite aggressive and have lymph node metastasis at the time of diagnosis.
Adenosquamous carcinoma presents as a palpable mass and has been found in women whose age ranges from 31 to 87 y [5]. At gross examination, low-grade adenosquamous carcinomas tend to display a stellate or infiltrative configuration, with poorly defined borders. Microscopically, the carcinomatous component is characterized by small glandular structures, with rounded rather than angulated contours, and solid cords of epithelial cells, which may contain squamous cells, squamous pearls or squamous cyst formation. The invasive neoplastic component typically shows long, slender extensions at the periphery and infiltrate in between normal breast structures, features which have been associated with inadequate local excision and high incidence of recurrence [1]. Clusters of lymphocytes are often observed at the periphery. Furthermore, the association between these tumours and adenomyoepithelioma and sclerosing proliferative lesions has been reported [6,7].
Adenosquamous carcinoma is consistently negative for ER, PR, and HER2-neu expression, hence may be a useful diagnostic tool. Myoepithelial and cytokeratin stains are positive, but the extent of staining is highly variable. SMA, p63, calponin, and CD10 show variable degree of positivity [8,9].
These tumours with negative ER, PR, HER2 receptors are classified under “basal-like” subtype of molecular classification. They have prognostic significance. These tumours have distinct genetic and epidemiological features. These tumours are high grade and have a high proliferation rate. They exhibit frequent metastasis to viscera and brain. They are not responsive to endocrine therapy but respond to platinum based chemotherapy.
According to study conducted by Geyer et al., [9] who observed five cases of adenosquamous carcinoma of breast, all of them belonged to 54-76 y, in contrast our case was 49-years-old. In their study, all cases showed negative axillary nodes. Similarly, our case also showed negative axillary lymph nodes.
Conclusion
This case is presented for its rare gross appearance as solitary cystic lesion and its microscopic appearance as adenosquamous variant of metaplastic carcinoma of breast. This variant should be identified for the purpose of its biological behaviour.
* ER- estrogen receptor, PR- progesterone receptor, HER2- Her2neu ,** CK- cytokeratin
[1]. Tavassoli FA, Devilee P, Tumours of the Breast. International Agency for Research of Cancer (IARC) 2003 Lyon, France [Google Scholar]
[2]. Weigelt B, Reis-Filho JS, Histological and molecular types of breast cancer: is there a unifying taxonomy?Nat Rev Clin Oncol 2009 6:718-30. [Google Scholar]
[3]. Woodard BH, Brinkhous AD, McCarty KS Jr, Adenosquamous differentiatin in mammary carcinoma: an ultrastructural and steroid receptor onlyArch Pathol Lab Med 1980 104(3):130-33. [Google Scholar]
[4]. Rosen PP, Ernsberger D, Low grade adenosquamous carcinoma.A variant of metaplastic mammary carcinomaAm J Surg Pathol 1987 11:351-58. [Google Scholar]
[5]. Flecthur C, Diagnostic Histopathology of Tumour 2007 7th edPhiladelphiaChurchill Livingstone Elsevier [Google Scholar]
[6]. Van Hoeven KH, Drudis T, Cranor ML, Low-grade adenosquamous carcinoma of the breast. A clinocopathologic study of 32 cases with ultrastructural analysisAm J Surg Pathol 1993 17:248-58. [Google Scholar]
[7]. Denley H, Pinder SE, Tan PH, Metaplastic carcinoma of the breast arising within complex sclerosing lesion: a report of five casesHistopathology 2000 36:203-09. [Google Scholar]
[8]. Kawaguchi K, Shin SJ, Immunohistochemical staining characteristics of low-grade adenosquamous carcinoma of the breastAm J Surg Pathol 2012 36:1009-20. [Google Scholar]
[9]. Geyer FC, Lambros MB, Natrajan R, Mehta R, Mackay A, Savage K, Genomic and immunohistochemical analysis of adenosquamous carcinoma of the breastMod Pathol 2010 23:951-60. [Google Scholar]