Metastatic Amelanotic Melanoma with Occult Primary Masquerading as Sarcoma
Niyaz Ahmed1, Ranjit Kumar Padhiari2, Praveen G P3, Vishnu Kurpad4
1 Senior Resident, Department of General Surgery, Bangalore Medical College and Research Institute, Bangalore, India.
2 PG Student, Department of General Surgery, Bangalore Medical College and Research Institute, Bangalore, India.
3 PG Student, Department of General Surgery, Bangalore Medical College and Research Institute, Bangalore, India.
4 Resident, Department of Oncosurgery, Kidwai Memorial Institute of Oncology, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Niyaz Ahmed, Devinagar, Door. No. 25, Ward No. 25, Near Mecca Mosque, Bellary, Karnataka- 583104, India.
E-mail: niyazbly8940@gmail.com
Malignant melanoma with occult primary is extremely rare. It is found that survival is almost same or even better than the melanomas with known primary site. Surgeons should have a high index of suspicion when a patient presents like sarcoma which bleeds profusely when planning for excision. Here, is an unusual case of young adult which presented initially with granulomatous lymphandenitis in axilla with primary suspicion of tuberculosis, later turning out to be sarcoma on FNAC and MRI. On immunochemistry (IHC), the final diagnosis of amelanotic melanoma was made and further workup did not show up any primary site of origin.
FNAC, Granulomatous lymphadenitis, Sarcoma
Case Report
A 24-year-old male presented with huge swelling in right axilla of 12 x 7cms, irregular, hard in consistency, fixed to underlying structures with normal skin over the swelling clinically diagnosed as soft tissue sarcoma [Table/Fig-1].
Initial presentation as large axillary mass clinically resembling soft tissue sarcoma

One year ago, patient had presented with a right axillary swelling, 2 x 1.5 cm, freely mobile, non-tender and firm in consistency. Thinking it as a lymphnode, excision biopsy was planned. Due to profuse bleeding complete excision could not be performed and the obtained tissue of swelling was sent for Histopathological examination (HPE) which showed chronic non-specific inflammatory lesion and a course of antibiotics was given. The axillary swelling increased in next 6 months. USG axilla showed multiple axillary discrete lymphadenopathy with central necrosis. FNAC done at that time showed chronic granulomatous lymphadenitis suggestive of Koch’s [Table/Fig-2]. Chest x-ray was normal, Montaux negative but Anti TB-IgM was 1.0 (positive >1, borderline 0.2-0.8). A provisional diagnosis of TB lymphadenitis was made and ATT was started, but patient defaulted after a month’s medication due to no improvement and continuous increase in size of the swelling. Finally presented to us with a huge axillary swelling with previous treatment data. Repeat FNAC showed spindle to oval cells with prominent nucleoli, positive for malignant cells s/o Spindle cell sarcoma [Table/Fig-3].
Excision biopsy of the axillary lymph node done one year ago showing features s/o granulomatous lymphadenitis

FNAC of the axillary lesion showing spindle cells suggestive of sarcoma

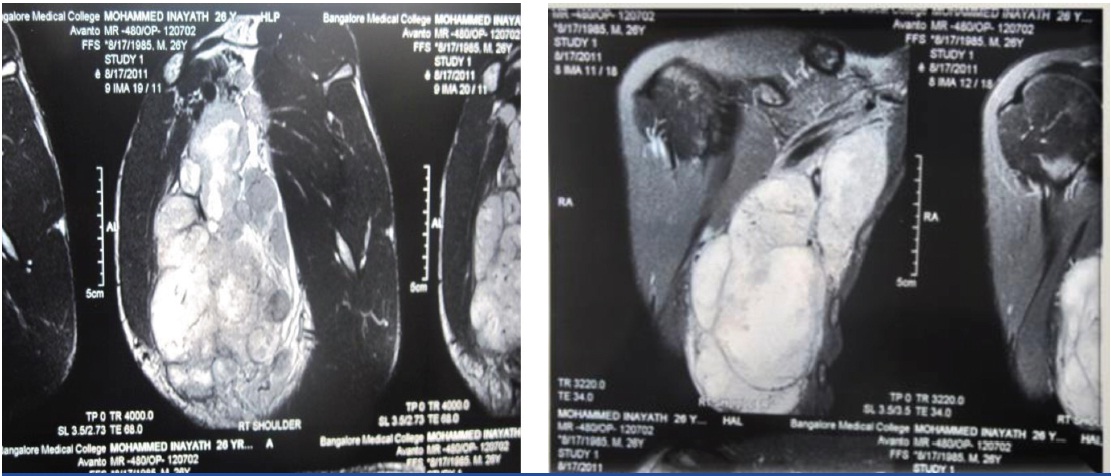
MRI scan of the right shoulder region showed the extent of the lesion and its relation with surrounding structures [Table/Fig-4&5].
MRI axilla showing the extent of suspected soft tissue sarcoma,
Patient underwent wide local excision of the lesion with level 3 axillary lymph node dissection and latissimus dorsi flap reconstruction [Table/Fig-6].
View during surgery after wide local excision. Axillary vein free of tumor preserved

Histopathological examination showed malignant spindle cell tumour [Table/Fig-7], with overlying skin free of tumour morphologically favouring Synovial sarcoma. IHC was done and was strongly positive for S-100 [Table/Fig-8], Vimentin, Melan-A, HMB-45, EMA s/o metastatic malignant melanoma. Fonatana-Masson staining did not reveal any melanin pigment. A final diagnosis of metastatic amelanotic melanoma was made. No primary was found even after extensive search. Bronchoscopy, endoscopy and colonoscopy revealed no lesions. Fundus examination was normal. Whole body PET CT was done [Table/Fig-9]. It showed a metabolically active right neck level IV lymph node, likely metastatic and irregular patchy opacity of 1.3 x 1.5cm in left lung apex. Patient underwent Radical neck dissection with thoracotomy and the lesions were sent for HPE. Lung lesion showed fibrotic tissue, and neck node metastatic. Patient refused for chemotherapy and expired after one year due to metastasis.
Histopathology of the resected specimen shows f/s/o spindle cell variant of soft tissue sarcoma

IHC staining showing s-100 positive

Follow up PET CT 6 months after surgery showing hot spots in neck and left lung apex. FIGURE-9. IHC staining showing s-100 positive

Discussion
Malignant melanoma is common neoplasm of melanocytes. Tumour is prone to metastasize early with lymph node metastasis presenting earlier than haematogenous. Melanoma presenting with unknown primaries are rare [1]. Limited data is available regarding presentation of metastases of malignant melanoma simulating soft tissue sarcoma to an extent which seems not to be recognised [2]. No data is found regarding presentation of secondaries in occult malignant melanoma as granulomatous lymphadenitis. Melanoma has a wide spectrum of histologic features which mimic epithelial, hematologic, mesenchymal, and neural tumour’s [3].
In the national cancer database [Table/Fig-10], the various presentations of melanoma are [1]:
National cancer database for various presentations of melanoma
| Site | Percentage % |
|---|
| Cutaneous | 91.2 |
| Ocular | 5.3 |
| Mucosal | 1.3 |
| Unknown primary site | 2.2 |
Metastatic amelanotic melanoma with unknown primary is a rare lesion and the incidence has been variously reported to be about 2.2% [1]. Metastasis of malignant melanoma simulating soft tissue sarcoma is also reported in literature [3]. Early detection of malignant melanoma has a cure rate of 87% [4] but majority present in a late stage. Status and number of lymph nodes involved is the single most important prognostic factor for survival. For unknown primary melanomas, the distribution of metastases as localized to a region or multiple sites at presentation was 43.0% and 57.0%, respectively [5]. Metastasis in a lymph node with occult primary may be due to spontaneous regression of a primary or a de novo origin from lymph node and soft tissues and regression is not an important factor predicting survival/metastasis [3,4]. A thorough physical and clinical examination including oral cavity, nasopharynx, GIT, genitalia, oral mucosa, anus, rectum and ocular examination and the draining lymph node areas is very crucial. Metastatic workup includes endoscopy, bronchoscopy, colonoscopy, FNAC of the lesion, fundoscopy. A CT brain to rule out secondaries in brain and CT thorax for lung secondaries [4]. FNAC for metastatic melanoma was found to have an overall sensitivity of 92.1% and a specificity of 99.2% [4,6]. PET has a clinically useful role in evaluating patients with Stage III malignant melanoma with distant metastasis [2,7].
Primary and metastatic malignant melanoma may assume histological pattern which makes the light-microscopic identification of the tumours difficult, or even impossible especially when they are amelanotic cells which mimic those of sarcoma or carcinoma [1,8]. Most important the investigation to distinguish melanomas from other tumours is immunohistochemistry. Markers like S-100, HMB-45, MART-1/Melan-A, Tyrosinase & MITF have been sensitive in diagnosing melanoma. But S-100 remains the most sensitive for melanocytic lesions [1,3,9].
Survival rates are better with regional disease in patients with malignant melanoma of unknown primary site when treated with radical surgical excision than patients with regional disease having known and excised primary site [1,3,9].
The various modalities of treatment for lymph node metastasis includes therapeutic lymph node dissection followed by radiotherapy which is of limited use. Melanoma is regarded as relatively chemotherapy-refractory tumour. Amelanotic melanoma with unknown primary generally respond well to cisplatin based chemotherapy [1]. Other chemotherapy agents used commonly are Dacarbazine, Fotemustine (nitrosourea). High dose interferon-alpha is the only agent to have gained FDA approval for the treatment of advanced disease [1]. Other options like Tamoxifen, IL-2, and combinations of biologic agents with chemotherapy drugs have been tried in advanced disease. After the onset of symptoms of metastatic malignant melanoma overall median survival and 5yr survival rate is 13%. 5yr disease free survival in patients with head & neck lesions is 25%. And those with lymphnode involvement, the survival rate is 18% [1,9].
In our case after wide surgical excision patient was advised for cisplatin based chemotherapy, which he refused. Patient expired after one year due to metatstasis.
Conclusion
Metastatic amelanotic melanoma with unknown primary is a rare lesion. The purpose of this article is to make doctors aware of the existence of a combination, albeit rare, of two features in a melanoma. Occult primary with metastasis in lymph nodes presenting as chronic granulomatous lymphadenitis and Metastasis from occult primary melanoma mimicking soft tissue sarcoma. FNAC and even histopathology of the resected specimen can mislead the diagnosis of Malignant Melanoma. Confirming diagnosis with IHC is vital for proper multimodal management of the this rare disease condition.
[1]. Slingliff Craig L jr, Flaherty Keith, Rosenberg Steven A, Read Paul W De Vita, Hellman, and Rosenberg’sCancer Principles and Practice of Oncology 2011 119:1643-87. [Google Scholar]
[2]. Reinhardt Micheal J, Joe Alexius Y, Jaegaer Ursula, Diagnostic performance of whole body dual modality 18F-FDG PET/CT Imaging for N- and M- staging of malignant melanoma : experience with 250 consecutive patientsJCO 2006 24(7):1178-87. [Google Scholar]
[3]. Lodding Par, Kindblom Lars-Gunnar, Angervall Lennart, Metastatis of malignant melanoma simulating soft tissue sarcomaVirchoues Arch A Pathol and Histopathol 1990 417:377-88. [Google Scholar]
[4]. Kelly M, McMaster Urist Marshall M, Melanoma and cutaneous malignanciesSabiston textbook of surgery3219th edi:742-67. [Google Scholar]
[5]. Chang AE, Karrnell LH, Menck HR, The national cancer database report on cutaneous and non cutaneous melanomasCancer 1998 83:1664-78. [Google Scholar]
[6]. Doubrovsky Anna, Scolyer Richard A, Murali Rajmohan, Diagnostic accuracy of fine needle biopsy for metastatic melanoma and its implications for patient managementAnn Surg Oncol 2008 15(1):323-32. [Google Scholar]
[7]. Tyler DS, Onaitis M, Khernai A, Hata A, Positron emission tomography scanning in malignant melanomaCancer 2000 89:1019-25. [Google Scholar]
[8]. Siddaraju N. Clinical cytology in the diagnosis and management of melanoma, current management of malignant melanoma, Dr. Ming Yu Cao(Ed.), 2011. ISBN:978-953-307-264-67 [Google Scholar]
[9]. Lewis Karl D, Dollarhide Susan RN, James E, Metastatic malignant melanoma from an unknown primary presenting as a large axillary massOncology 2006 20(7):763-70. [Google Scholar]