Periodontitis is a chronic inflammatory response to the subgingival bacteria, producing irreversible periodontal tissue destruction and tooth loss. Periodontopathic bacteria stimulate cells comprising periodontal tissues to express various inflammatory cytokines. These cytokines may activate the production of matrix metalloproteinases (MMPs), prostaglandin E2 (PGE2) and the differentiation of osteoclasts, resulting in connective tissue destruction and alveolar bone resorption.
However, tissue destruction could be protected by suppressing the activity of proinflammatory cytokines by anti-inflammatory cytokines such as TGF-β1, which is predominantly produced by T regulatory (Treg) cells and macrophages and could also induce a wide range of essential functions including activation, proliferation, migration and the synthesis of extracellular matrix (ECM) components [1].
Transforming Growth Factor-β1 has highly diverse biological effects including the chemotactic and the mitogenic activity of gingival and periodontal ligament fibroblasts, and the upregulation of ECM components including collagen, fibronectin, tenascin and proteoglycans. The precise role of TGF-β1 in periodontal wound healing remains unclear. It has also been found that TGF-β1 has some degree of clinical efficacy in promoting periodontal regeneration [2].
The present study aimed to assess the changes in levels of Transforming Growth Factor– β1 (TGF-β1) in gingival crevicular fluid (GCF) in sites with chronic periodontitis at various time intervals before and after periodontal surgery.
The correlation of TGF-β1 levels and periodontal parameters: such as probing pocket depth (PPD) and clinical attachment level (CAL) before and after periodontal surgery.
Materials and Methods
A total of 18 sites around anterior teeth from adult subjects with chronic periodontitis were selected for the study from the outpatient section, Department of Periodontology, Meenakshi Ammal Dental College, Chennai. Six sites (mesio-buccal, mid-bucccal, disto-buccal, mesio-palatal, mid-palatal, disto-palatal) were examined around periodontally compromised anterior teeth. The site exhibiting the deepest probing pocket depth and clinical attachment level at baseline was selected for the study. GCF sampling and periodontal parameters recording was done from this selected site at various time intervals of the study.
The study was approved by Meenakshi Institutional Ethics Committee Clearance Board. All patients were explained about the study and written informed consent was obtained from those who agreed to voluntarily participate in this study.
Sites affected with chronic periodontitis were given non-surgical and surgical therapy and the effects of therapy on periodontal parameters and TGF-β1 levels in the GCF were assessed upto six weeks post-treatment.
Inclusion Criteria
Sites with probing pocket depth (PPD) of ≥ 5 mm
A lifetime cumulative attachment loss (LCAL) of ≥ 5mm.
Exclusion Criteria
Subjects with aggressive forms of periodontitis.
History of diabetes mellitus.
Pregnancy.
Subjects who need antibiotic prophylaxis.
Subjects taking steroids, anti-inflammatory drugs, or antibiotics (in the last 6 months).
Smoking and alcoholism.
Anomalies of blood and immune system.
Subjects who have received any periodontal treatment in the last six months.
Treatment Protocol
Periodontal parameters assessed at all sites at various time intervals were PPD and CAL.
GCF was collected using extrasulcular method from all selected sites at various time intervals for TGF-β1 estimation.
Baseline – Clinical evaluation: A brief case history was recorded which included patient’s chief complaint, medical and dental history, oral examination and routine radiographic examination, following which periodontal parameters were recorded.
Patients recalled the next day for supragingival scaling followed by GCF collection.
One week later – Scaling and root planing done for the quadrant containing the selected sites.
Pre-Surgery – GCF collection was done followed by measurement of periodontal parameters at the selected sites. Periodontal flap surgery performed for the same quadrant.
Two Weeks Post-Surgery – Only GCF collection done from the selected sites.
Six Weeks Post-Surgery - GCF collection done and periodontal parameters recorded from the selected sites.
GCF collection
The selected site was air dried and isolated with cotton rolls. A standardised volume of 1 μl GCF was collected from the selected sites in Eppendorf tubes using black colour-coded 1-5μl calibrated volumetric microcapillary pipettes (Sigma-Aldrich Chemical Company, USA.) by placing the tip of the pipette extracrevicularly (unstimulated) for 5-20 min.
Micropipettes contaminated with blood or saliva was discarded. GCF samples were stored at -70°C till the time of assay.
The concentration of TGF-β1 in the GCF samples was determined using a human TGF-β1 enzyme immunometric assay (EIA) kit. (DRG Instruments GmbH, Germany).
Surgical Procedure
The surgical area was anesthetized using 2% lidocaine hydrochloride with adrenaline (1:2,00,000). Sulcular incisions were made using a no. 15 Bard-Parker blade and mucoperiosteal flaps were elevated at the buccal and lingual/palatal aspects. A thorough debridement of the affected sites was done and the exposed root surfaces were thoroughly planed to a smooth hard surface. The flaps were approximated and a total soft tissue primary closure was obtained by using 4-0 sutures in interrupted fashion.
No medication was prescribed for all patients at various stages of treatment during the course of the study. Since antibiotics and analgesics tend to interfere with the immune and inflammatory gingival tissue responses following tissue destruction and repair, medication was avoided to accurately assess the specific action of anti-inflammatory cytokines such as TGF-β1 in suppressing the activity of proinflammatory cytokines during tissue repair. All patients were prescribed 0.2% chlorhexidine mouthwash twice daily for two weeks for effective plaque control following surgery.
Statistical Analysis
Mean and Standard deviation for TGF-β1 values, probing pocket depth (PPD) and clinical attachment level (CAL) were taken at Baseline, Pre-surgery, and two Weeks Post-surgery and six Weeks Post-surgery. Paired samples t-test was applied for comparison of TGF-β1 values, probing depth (PD) and clinical attachment level (CAL) between two time points.
One-way ANOVA F-test was used to calculate the p-value. p-value < 0.05 was considered statistically significant.
Results
TGF-β1 was detected in all samples collected at different time intervals during the course of the study. [Table/Fig-1] shows the mean TGF-β1 levels detected at different time intervals. [Table/Fig-2] shows the correlation between TGF-β1 levels at various time points. There was significant reduction in TGF-β1 levels at the Pre-surgery, two Weeks Post-surgery and six Weeks Post-surgery intervals when compared to Baseline levels (p-value<0.05). The difference in TGF-β1 levels between the Pre-surgery and two Weeks Post-surgery interval (p-value=0.596), Pre-surgery and six Weeks Post-surgery interval (p-value=0.213) and two Weeks post op and six Weeks Post-surgery interval (p-value=0.217) were not significant.
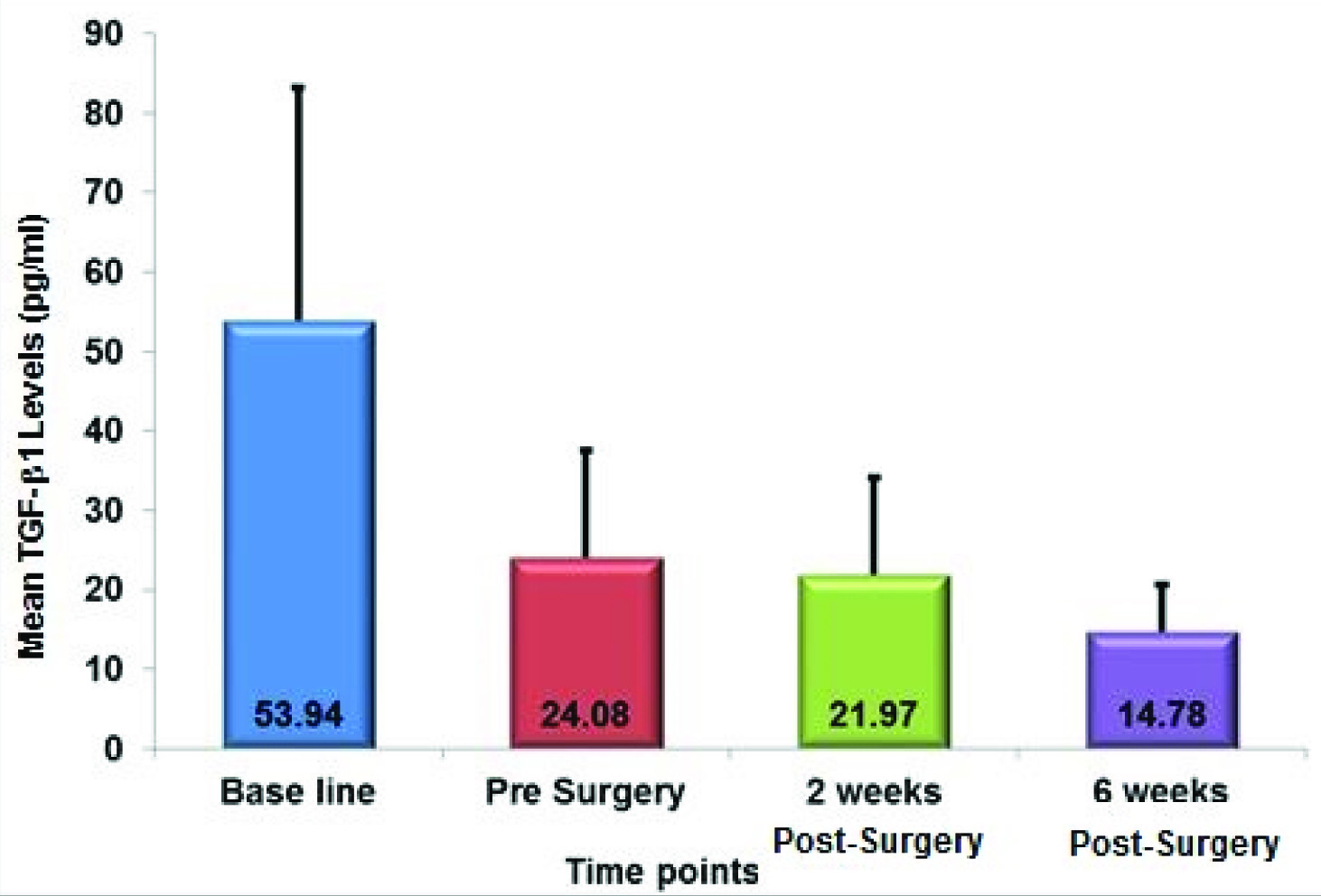
Correlation between TGF-β1 Levels at Various Time Points
| Time Points | N | Mean (pg/ml) | S.D. | p-Value |
|---|
| TGF-β1 – Baseline | 18 | 53.943 | 29.248 | |
| TGF-β1 - Pre Surgery | 24.084 | 13.380 | |
| Diff between TGF-β1 conc. at Baseline to Pre Surgery | 29.859 | 26.631 | 0.040 |
| TGF-β1 - Baseline | 18 | 53.943 | 29.248 | |
| TGF-β1 - 2 weeks Post-Surgery | 21.967 | 12.087 | |
| Diff between TGF-β1 conc. at Baseline to 2 Weeks Post-Surgery | 31.977 | 23.93 | 0.022 |
| TGF-β1 – Baseline | 18 | 53.943 | 29.248 | |
| TGF-β1 - 6 weeks Post-Surgery | 14.779 | 5.963 | |
| Diff between TGF-β1 conc. at Baseline to 6 Weeks Post-Surgery | 39.164 | 26.868 | 0.016 |
| TGF-β1 - Pre Surgery | 18 | 24.084 | 13.380 | |
| TGF-β1 - 2 weeks Post-Surgery | 21.967 | 12.087 | |
| Diff between TGF-β1 conc. at Pre Surgery to 2 Weeks Post-Surgery | 2.118 | 9.172 | 0.596 |
| TGF-β1 - Pre Surgery | 18 | 24.084 | 13.380 | |
| TGF-β1 - 6 weeks Post-Surgery | 14.779 | 5.963 | |
| Diff between TGF-β1 conc. at Pre Surgery to 6 Weeks Post-Surgery | 9.306 | 15.984 | 0.213 |
| TGF-β1 - 2 weeks Post-Surgery | 18 | 21.967 | 12.087 | |
| TGF-β1 - 6 weeks Post-Surgery | 14.779 | 5.963 | |
| Diff between TGF-β1 conc. at 2 Weeks Post-Surgery to 6 Weeks Post-Surgery | 7.188 | 12.469 | 0.217 |
[Table/Fig-3] shows the mean PPD recorded at different time intervals. [Table/Fig-4] shows the correlation between probing pocket depth (PPD) at various time points and there was a significant reduction in PPD among all intervals (p-value<0.05) throughout the course of the study.
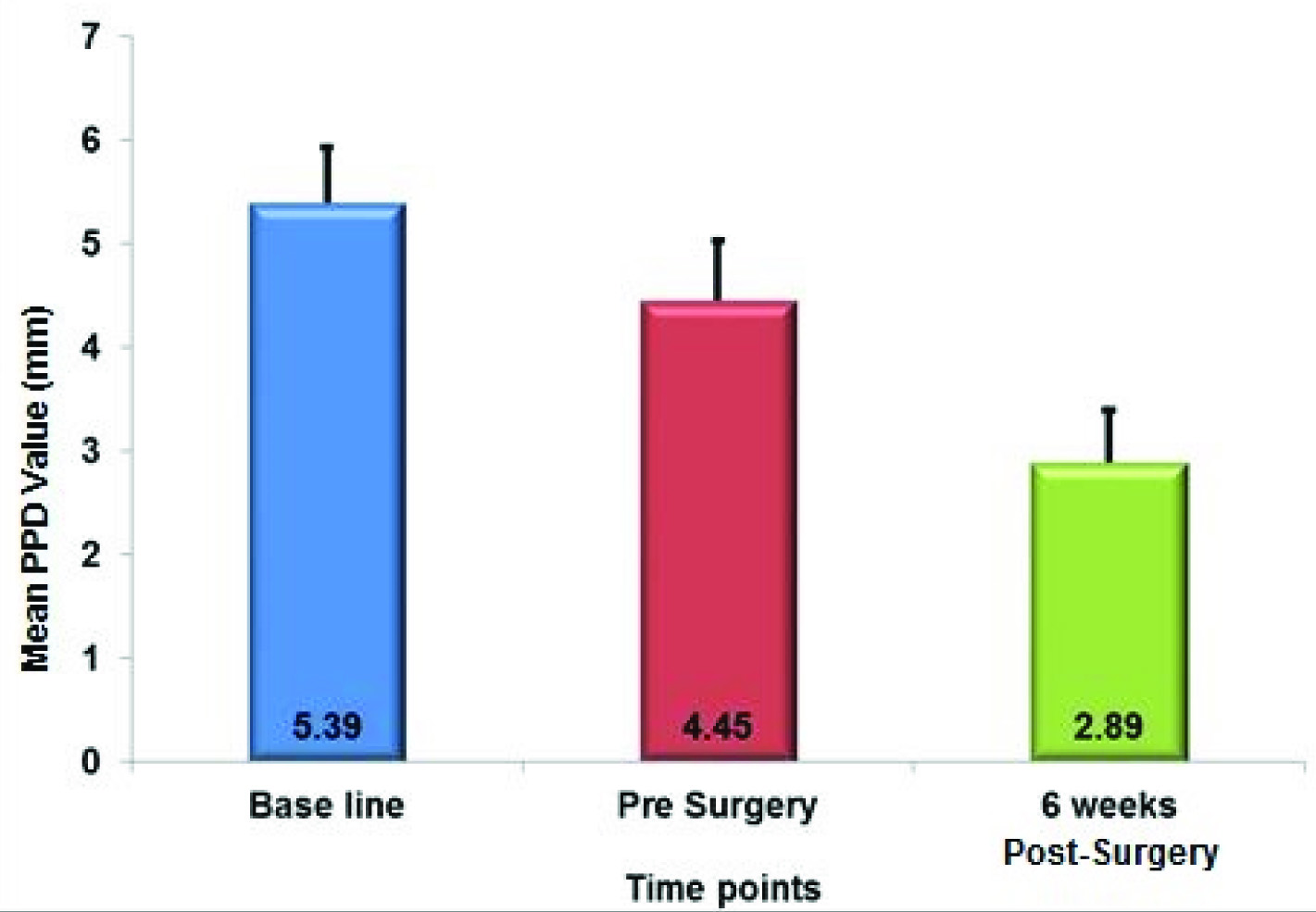
Correlation between Probing Pocket Depth (PPD) at Various Time Points
| Time Points | N | MEAN (mm) | S.D. | p-Value |
|---|
| PPD - Baseline | 18 | 5.392 | 0.537 | |
| PPD - Pre Surgery | 4.448 | 0.585 | |
| Diff between PPD at Baseline to Pre Surgery | 0.943 | 0.492 | <0.005 |
| PPD - Baseline | 18 | 5.392 | 0.537 | |
| PPD - 6 Weeks Post-Surgery | 2.893 | 0.502 | |
| Diff between PPD at Baseline to 6 Weeks Post-Surgery | 2.498 | 0.184 | <0.001 |
| PPD - Pre Surgery | 18 | 4.448 | 0.585 | |
| PPD - 6 Weeks Post-Surgery | 2.893 | 0.502 | |
| Diff between PPD at Pre Surgery to 6 Weeks Post-Surgery | 1.555 | 0.455 | <0.001 |
[Table/Fig-5] shows the mean CAL recorded at different time intervals. [Table/Fig-6] shows the correlation between clinical attachment level (CAL) at various time points and there was a significant gain in CAL among all intervals (p-value<0.05) throughout the course of the study.
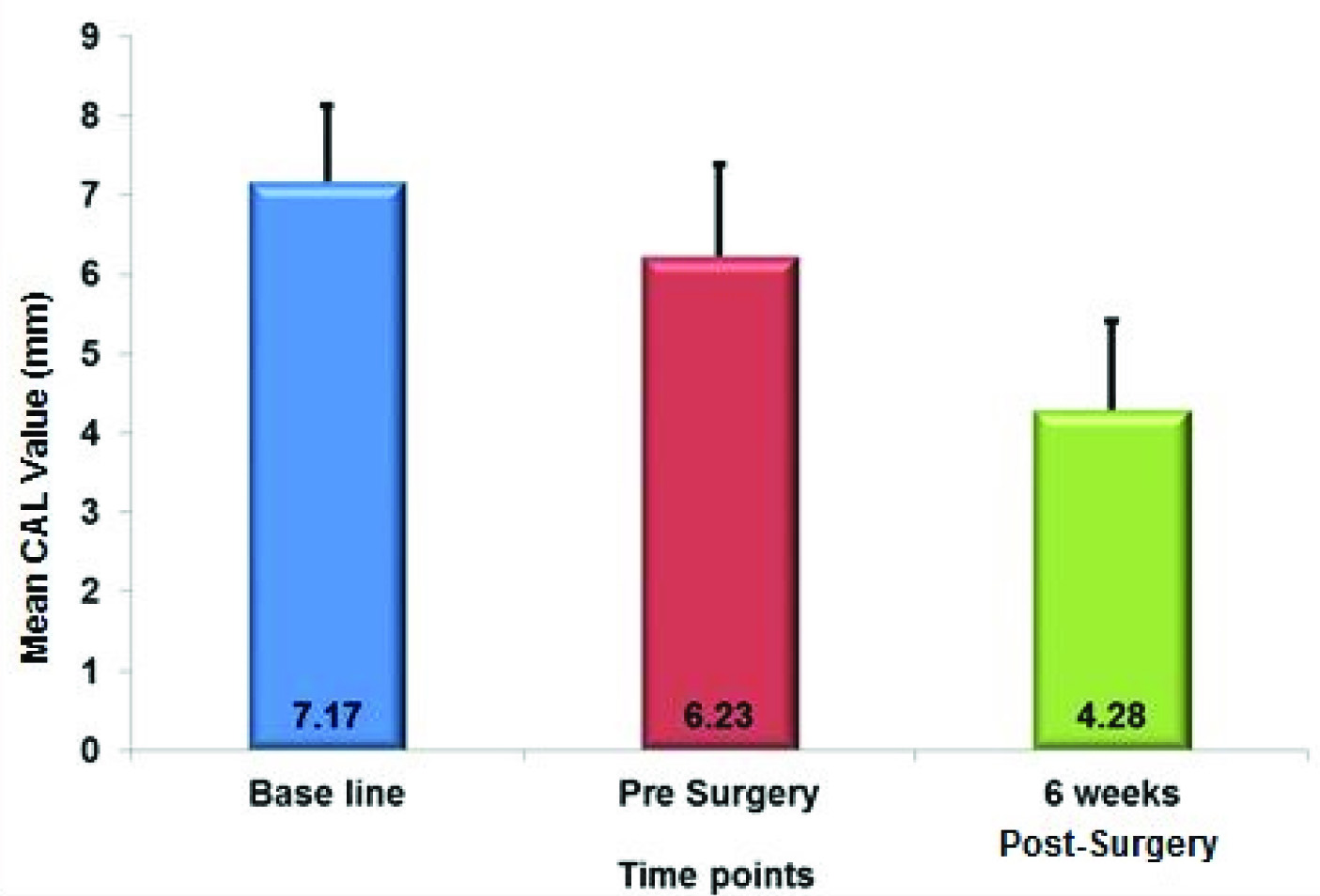
Correlation between Clinical Attachment Level (CAL) at Various Time Points
| Time Points | N | Mean (Mm) | S.D. | P-Value |
|---|
| CAL – Baseline | 18 | 7.170 | 0.959 | |
| CAL - Pre Surgery | 6.225 | 1.168 | |
| Diff between CAL at Baseline to Pre Surgery | 0.945 | 0.391 | <0.002 |
| CAL – Baseline | 18 | 7.170 | 0.959 | |
| CAL – 6 Weeks Post–Surgery | 4.282 | 1.126 | |
| Diff between CAL at Baseline to 6 Weeks Post-Surgery | 2.888 | 0.406 | <0.001 |
| CAL - Pre Surgery | 18 | 6.225 | 1.168 | |
| CAL - 6 Weeks Post–Surgery | 4.282 | 1.126 | |
| Diff between CAL at Pre Surgery to 6 Weeks Post-Surgery | 1.943 | 0.441 | <0.001 |
Discussion
The present study aimed to assess the changes in levels of Transforming Growth Factor– β1 (TGF-β1) in GCF in sites with chronic periodontitis at various time intervals before and after periodontal surgery and to study the correlation of TGF-β1 levels and periodontal parameters: such as probing pocket depth (PPD) and clinical attachment level (CAL) before and after periodontal surgery.
GCF was used for assessment of TGF-β1 levels since although GCF has been shown to originate from the serum in proximal blood vessels, it is generally considered to reflect the ongoing processes in the surrounding periodontal tissues, including inflammation, turnover of connective tissue and resorption of alveolar bone [3]. Collection of GCF using micropipettes appears to be ideal as it provides an undiluted sample of ‘native’ GCF whose volume can be accurately assessed [4]. Only the anterior teeth were included in the study to improve access and to reduce the risk of salivary contamination during the process of GCF collection [5].
TGF-β1 could be detected in all the samples from sites with chronic periodontitis which was in accordance with a similar study done by Sattari et al., [1]. Steinsvoll et al., [6] had stated that TGF-β1 is an immunosuppressive cytokine that stimulates wound healing and up regulation of TGF-β1 in inflamed gingiva may counterbalance for destructive gingival inflammatory responses that are simultaneously taking place in patients with chronic marginal periodontitis which could explain the increased levels of TGF-β1 at Baseline in this study.
Various studies by Talonpoika et al., [7,8] have shown that newly synthesized connective tissue components such as collagen type I and III and fibronectin are present at elevated levels in the GCF of treated sites which could be the reason for presence of TGF-β1 in GCF during the initial stages Post-surgery in this study. Postlethwaite et al., [9] had stated that TGF-β1 is present during initial phases of wound healing which could explain how TGF-β1 was detected in all sites following treatment as well.
The precise role of TGF-β1 in periodontal wound healing remains unclear and studies done by Skaleric et al., [10] Ko et al., [11] and Gürkan et al., [12] have shown that concentration of TGF-β1 of gingival tissue exhibits dynamic changes associated with the progression of experimental periodontal inflammation and the levels of TGF-β1 in gingival tissue may be valuable in detecting the inflammatory reaction of periodontal tissues.
This study reported a decrease in GCF TGF-β1 levels at two weeks post-surgery which was not in accordance with the findings of Kuru et al., [2] who reported an initial increase in GCF TGF-β1 concentrations upto two weeks following surgery. However, the increase in TGF-β1 levels at two weeks post-surgery interval in his study was not statistically significant. In view of the clinical outcomes of periodontal surgery observed by various authors [10–12], it is likely that the GCF samples collected from surgically treated sites represent wound fluid associated with periodontal repair and regeneration and this could explain the varying levels of GCF TGF-β1 in the course of this study.
There was a significant decrease in GCF TGF-β1 concentrations from Baseline to 6 Weeks Post-surgery in this study and these findings are in agreement with those of Kuru et al., [2] and Sattari et al., [1]; this could be due to the elimination of the microbial factors which causes a gradual decrease in the level of bacterial toxins followed by decline in the production of inflammatory mediators and stimulants as well.
A study done by Skaleric et al., [10] established that low concentrations of TGF-β1 at the beginning of the inflammatory process stimulate chemotactic recruitment and activation of neutrophils, monocytes and lymphocytes, whereas in advanced gingival inflammation, TGF-βl stimulation is reversed. This may be considered as a feedback control mechanism for the progression of inflammation and explain the changes in GCF TGF-β1 levels at the various stages of periodontal health and disease during the course of this study.
Caffesse et al., [13] had stated that non-surgical treatment approach frequently results in insufficient root debridement especially at sites with deep pockets. Hence, all sites were subjected to SRP followed by open flap debridement in accordance with the study done by Kim et al., [14] Becker et al., [15] and Silva et al., [16] had stated that the reduction in probing pocket depth and gain in CAL was a result of reduction in pathogenic bacteria and conversion of inflamed gingiva to healthy gingiva with or without new connective tissue attachment which explains the improvement in periodontal parameters from Baseline to Pre-surgical time intervals. The improvement in periodontal parameters following treatment in this study were in accordance with a systemic review done by Heitz-Mayfield et al., [17] which established that in deep periodontal pockets (≥6 mm), surgical therapy resulted in more probing pocket depth reduction and more attachment gain than the non-surgical therapy.
Some limitations of this study were the small sample size (18 sites), short time interval observations and absence of a control group. Further long term studies with larger study subjects are required to understand the impact of various treatment modalities like oral prophylaxis, SRP and conventional flap surgery on controlling the periodontal inflammation and the positive influence of anti-inflammatory mediators like TGF-β1 in regulating the patient’s periodontal status. Since this was a prospective study with statistical comparison of select parameters over a set course of time, the requirement for a control group was not warranted and hence not included in the study design.
Conclusion
The results of the present study provide site-specific information on significant changes in TGF-β1 levels and periodontal parameters as a result of periodontal disease and treatment. Therefore, the present study indicates that TGF-β1 may play a role in the pathogenesis and diagnosis of periodontal disease and could be considered as a biomarker.