Non Traumatic Keratitis Due to Colletotrichum Coccodes: A Case Report
Aarti Kotwal1, Debasis Biswas2, Barnali Kakati3, Harsh Bahadur4, Neeti Gupta5
1 Associate Professor, Department of Microbiology, Himalayan Institute of Medical Sciences, Dehradun, Uttarakhand, India.
2 Additional Professor, Department of Microbiology, AIIMSBhopal, Saket Nagar, Bhopal, MP, India.
3 Associate Professor, Department of Microbiology, Himalayan Institute of Medical Sciences, Dehradun, Uttarakhand, India.
4 Professor, Department of Ophthalmology, Himalayan Institute of Medical Sciences, Dehradun, Uttarakhand, India.
5 Assistant Professor, Department of Ophthalmology, Himalayan Institute of Medical Sciences, Dehradun, Uttarakhand, India.
6 Lab Technician, Department of Microbiology, Himalayan Institute of Medical Sciences, Dehradun, Uttarakhand, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Debasis Biswas, Additional Professor, Department of Microbiology, AIIMS, Saket Nagar, Bhopal- 402624, MP, India. E-mail : dbiswas71@rediffmail.com
Colletotrichum species, a rare and emerging fungus is a well- known plant pathogen and an uncommon cause of human infection. It has been implicated as the etiological agent of cutaneous phaeohyphomycosis and keratitis, particularly following colonization of traumatized tissues or in immunocompromised patients. However, it has hardly ever been reported in the absence of such predisposing risk factors. Here, we report a case of keratitis with Colletotrichum coccodes occurring in a middle- aged, immunocompetent person without any history of trauma or co-morbidity. The isolate was sensitive to Amphotericin B and Voriconazole, and accordingly the patient was treated successfully with ocular administration of Amphotericin B.
Amphotericin, Ocular, Phaeohyphomycosis
Case Report
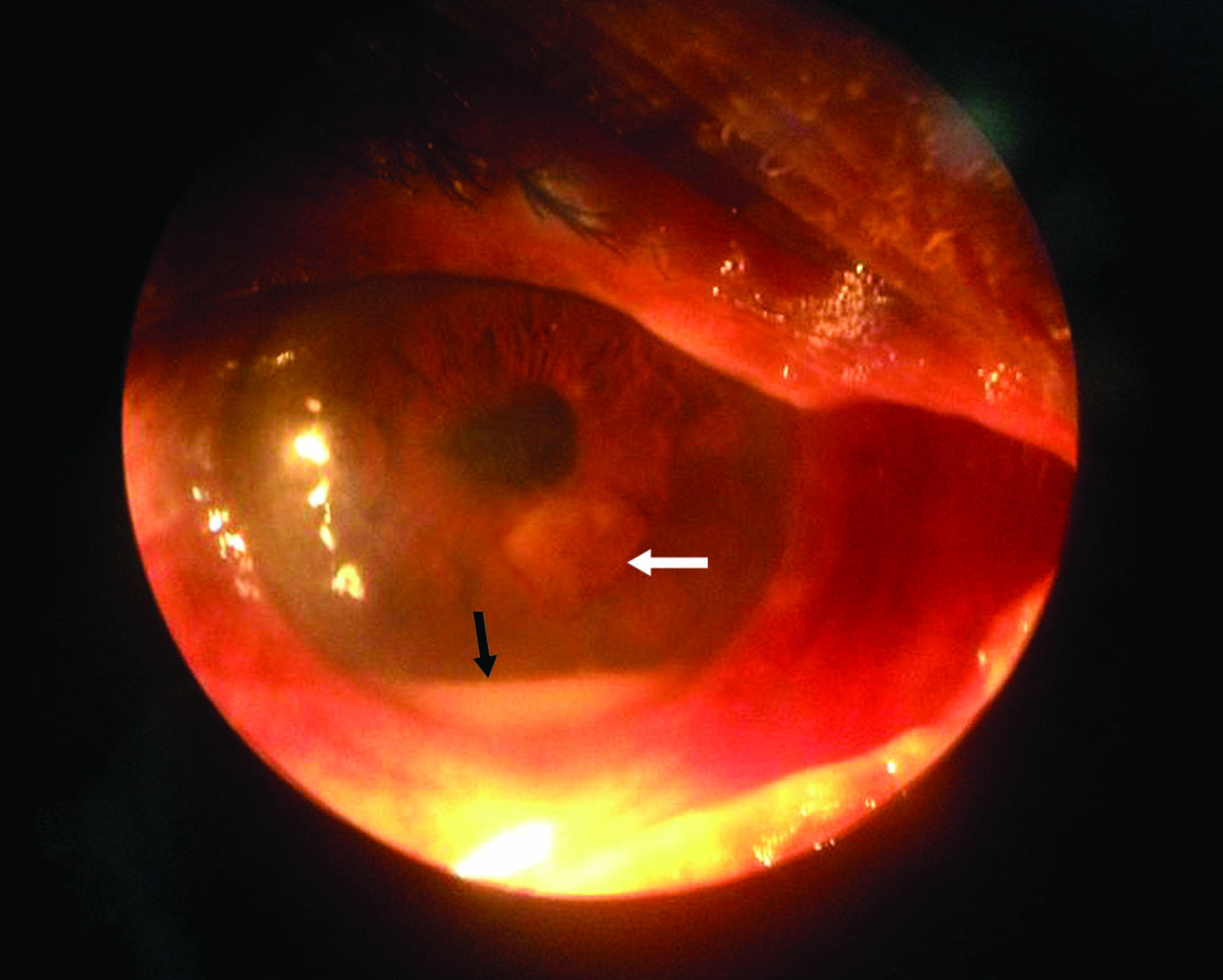
A 54-year-old male patient attended the Ophthalmology OPD of Himalayan Institute of Medical Sciences Dehradun in January 2012. An electrician by profession he complained of pain, mild watering, photophobia and gradual loss of vision in right eye. He was on topical Norfloxacin eye drops but without much relief since 1 month. Uncorrected visual acuity of the right eye was 6/60. On examination, mild oedema was observed in the upper and lower lids of the right eye. Slit lamp examination revealed a 4x5 mm sized, para-central corneal ulcer 5 mm from the limbus on the inferior nasal aspect [Table/Fig-1]. The ulcer was dry- looking with irregular margins and stromal infiltrates but there was no evidence of feathery margins. Satellite lesions were not seen and there was no evidence of immune ring. Infiltration involved all the layers of the cornea. There was an immobile hypopyon of 2 mm height from the limbus, present inferiorly. No pigmentation was noticed and lens and posterior segment were normal. Left eye was normal. A provisional diagnosis of fungal corneal ulcer was made. He did not reveal any history of trauma to the eye in the past.
Photograph of the affected eye showing the fungal ulcer (white arrow) and hypopyon (black arrow)
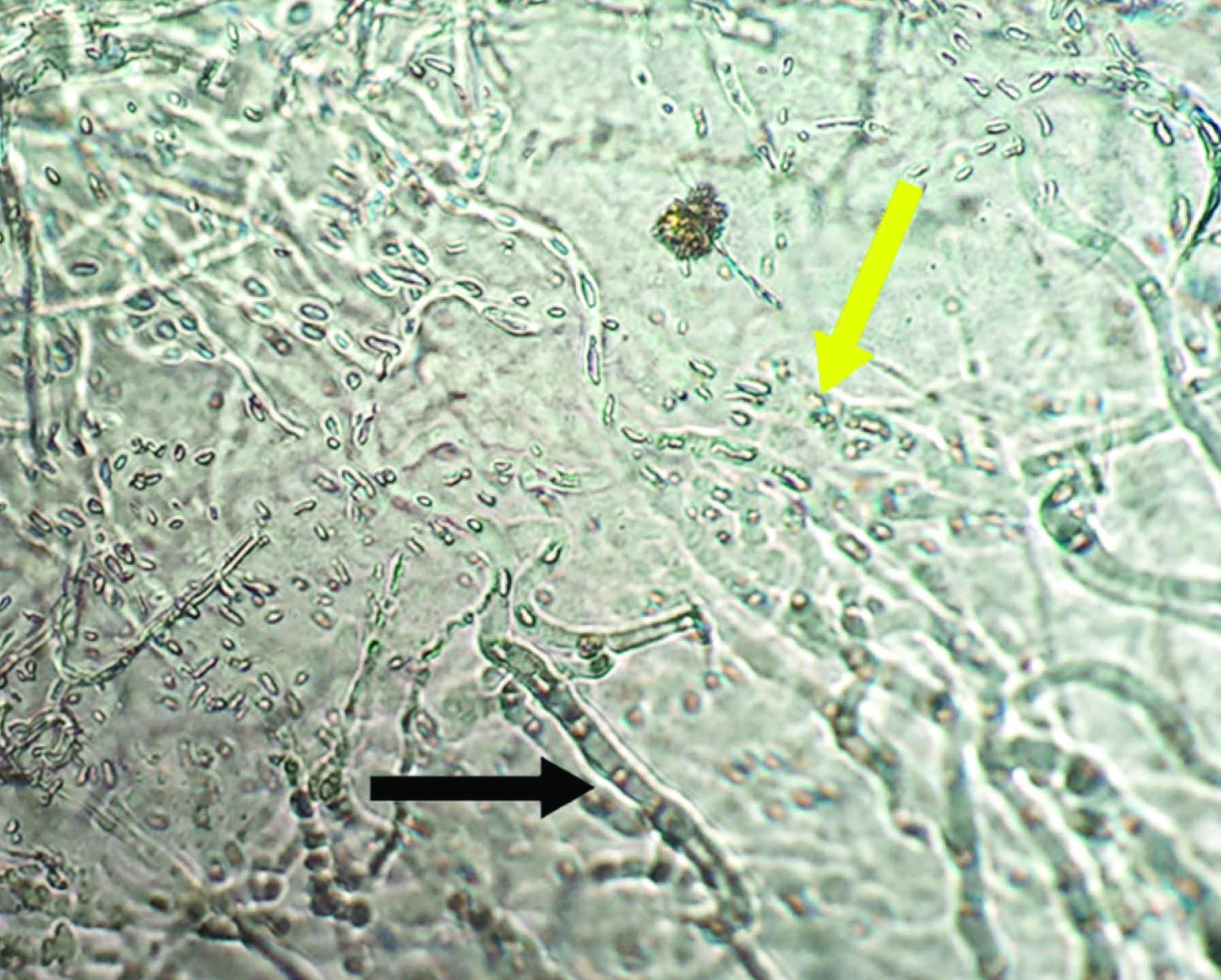
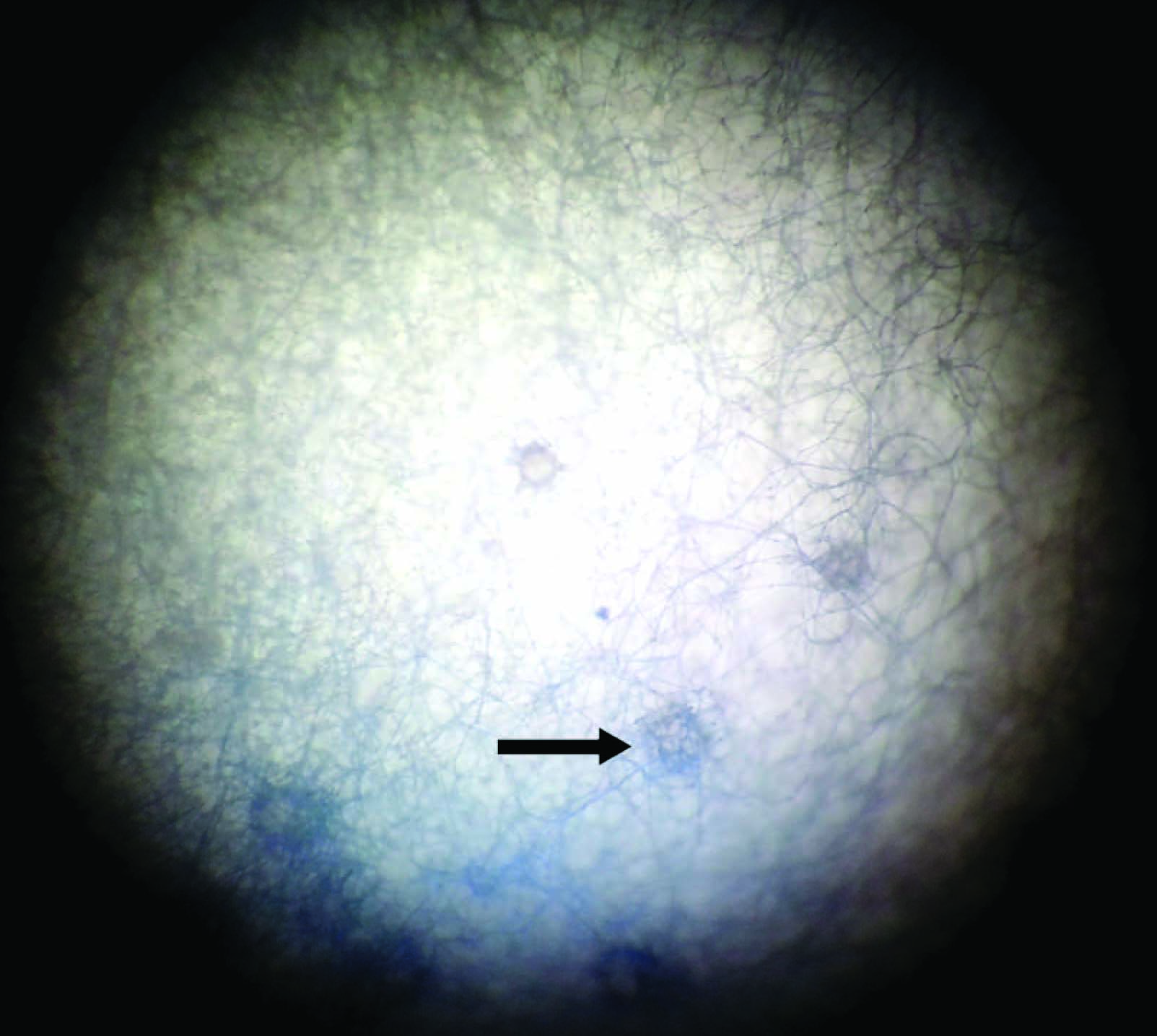
A direct KOH (Potassium hydroxide) mount from the corneal scraping revealed the presence of few septate hyaline hyphae. Growth on SDA (Sabouraud’s dextrose agar) was obtained within a week and greyish black mycelia with numerous black sclerotia and a brown colour on the obverse and reverse covering the whole of the culture tube was seen [Table/Fig-2a&b]. A LCB (Lactophenol cotton blue) tease mount from the growth on CMA (Corn meal agar) revealed acervular conidiomata with abundant setae, fusiform conidia and abundant appressoria with irregular margins [Table/Fig-3]. The slide cultures on PDA (Potato Dextrose Agar) after five days of growth at 25°C revealed hyaline, septate hyphae. Conidiomata were acervular, with 2 to 5 brown setae which had tapering ends [Table/Fig-4]. Long conidia which were hyaline, aseptate and fusiform with straight and parallel walls and abruptly tapered ends were seen. Also few light brown appressoria were found. Antifungal susceptibility testing was performed according to the Clinical and Laboratory Standards Institute guidelines(M38-A2). The antifungal agents used included Amphotericin B (10 U), Fluconazole (25 μg), Ketoconazole (10 μg) and Voriconazole (10 μg).The isolate was found to be sensitive to Amphotericin B and Voriconazole and the patient was treated successfully with ocular administration of Amphotericin B for 2 month. The patient was followed up regularly with no evidence of any reoccurrence.
a) Obverse image- Colonies of Colletotrichum coccodes as noted on cornmeal agar, b) Reverse image-Colonies of Colletotrichum coccodes as noted on cornmeal agar

Lactophenol cotton blue (LCB) mount of Colletotrichum coccodes showing the appresoria (black arrow) and fusiform nonseptate conidia (yellow arrow)
Acervuli containing setae (black arrow)
Discussion
We report for the first time the isolation of C. coccodes from India in a patient who had none of the known predisposing factors for this infection. He was successfully treated with ocular administration of Amphotericin B, contrary to the standard practice of surgical management for this condition.
Six of the 66 species of Colletotrichum have been implicated in human infections till date [1–4]. Though all of them have been isolated from eye infections world over, to the best of our knowledge, C. coccodes has been reported seldom from cases of keratitis [4–6]. Identification of the infecting species has therapeutic relevance, in view of species- specific antifungal susceptibility profile observed with Colletotrichum [7]. However, species identification, on the basis of morphological characteristics, is often difficult for this fungus [4]. Nutritionally deficient media are often helpful for the induction of sporulation. Apart from the commonly used nutritionally deficient media, viz. potato dextrose agar (PDA), we also obtained satisfactory results with the use of a different nutritionally deficient media, viz. corn meal agar (CMA). The close similarity between the curved conidia of Fusarium and Colletotrichum spp. presents a major difficulty for morphological identification. However, the presence of appressoria, nonseptate conidia and (in the later stages) acervuli with setae in Colletotrichum help in the discrimination. We speciated the isolate on the basis of the presence of sclerotia, appresoria with entire margins and fusiform conidia with abruptly tapered ends [8].
Being an emerging fungal pathogen in human diseases, the epidemiology of Colletotrichum is of considerable interest. Accordingly, speciation assumes importance for improved delineation of risk factors, clinical associations, transmission patterns and therapeutic outcomes. In addition, species-wise variation in the antifungal sensitivity profile has also been reported in Colletotrichum. While itraconazole has been reported to be ineffective in vitro against C. coccodes and C. dematium, it is active against some isolates of C. gloeosporioides [8]. Major degree of resistance to azoles among most strains of C. crassipes, C.gloeosporioides, C. coccodes and C. dematium have also been noted [7].
Though predominantly isolated in the context of preceding trauma [8] or underlying immunocompromising condition, the present case had none of these predisposing factors. Traumatic exposure, particularly to vegetable matter, has been suggested to impact the pathogenesis of this condition by initiating the release of conidia from the acervuli in the traumatized tissues.
Treatment of Colletotrichum keratitis is a combination of medical and surgical therapy [4]. In the review by Kaliamurthy et al., a complete resolution of Colletotrichum ulcers was reported by a combined medical and surgical approach [9]. Early removal of the fungal mass by keratoplasty or pars plans vitrectomy is the best surgical management option available till date [4]. There still exists a need to find an appropriate antifungal treatment of Colletotrichum keratitis and no consensus has been built on the choice of appropriate antifungal therapy for Colletotrichum ophthalmic infections. A good response to natamycin has been noted in patients despite exhibiting high minimum inhibitory concentrations (MIC) by in vitro susceptibility testing but Amphotericin B still remains the first choice for the treatment of these group of infections [2]. The antifungal susceptibility laboratory of the University of Texas corroborates these findings and reports a low MICs of Amphotericin B for most of their Colletotrichumspp [10]. In contrast, Shukla et al., have reported that Colletotrichum strains were more sensitive to Clotrimazole and Micanozole than Amphotericin B [11]. We performed antifungal susceptibility on our isolate and found that it to be resistant to Fluconazole. The isolate had low MICs to Amphotericin and Voriconazole. Our patient was started on topical Amphotericin B and successfully cured with complete healing of the ulcer and restoration of normal vision by two months.
One limitation of our study was that the morphological identification could not be confirmed by molecular methods like PCR amplification of the ITS region of the rRNA gene. We are currently aiming to archive Colletotrichum isolates recovered in our lab, in order to carry out molecular identification of the isolates in future.
Conclusion
The isolation of rare fungal pathogens from fungal keratitis, even in immunocompetent patients should not be underscored. Speciation and species-wise variation in the antifungal sensitivity profile of the isolates is decisive in the treatment of fungal keratitis.
[1]. Chakrabarti A, Shivaprakash MR, Singh R, Tarai B, George VK, Fomda BA, Fungal endophthalmitis: fourteen years’ experience from a centre in IndiaRetina 2008 28:1400-07. [Google Scholar]
[2]. De Hoog GS, Guarro J, Gene J, Figueras MJ, Atlas of clinical fungi: Central bureau voor schimmelcultures 1995 2nd edUtrecht, The Netherlands [Google Scholar]
[3]. Yegneswaran P, Pai V, Bairy I, Bhandary S, Colletotrichum graminicola keratitis: First case report from IndiaIndian J Ophthalmol 2010 58:415-17. [Google Scholar]
[4]. Shivaprakash M, Suma B, Dhaliwal M, Gupta A, Gupta S, Gupta A, Colletotrichum truncatum: an Unusual Pathogen Causing Mycotic Keratitis and EndophthalmitisJ Clin Microbiol 2011 49(8):2894-98. [Google Scholar]
[5]. Liesegang TJ, Forster RK, Spectrum of microbial keratitis in South FloridaAm J Ophthalmol 1980 90:38-47. [Google Scholar]
[6]. Natarajan SV, Rekha NS, Sharda RD, Mahalingam N, Colletotrichum keratitis: A Rare but Definite Clinical EntityJ Clin Diagn Res 2013 7(7):1430-33. [Google Scholar]
[7]. Guarro J, Svidzinski TE, Zaror L, Forjaz MH, Gene J, Fischman O, Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioidesJ Clin Microbiol 1998 36:3060-65. [Google Scholar]
[8]. Joseph J, Fernandes M, Sharma S, Colletotrichum dematium keratitisJ Postgrad Med 2004 50:309-10. [Google Scholar]
[9]. Kaliamurthy J, Kalavathy C, Ramalingam M, Prasanth D, Jesudasan C, Thomas P, Keratitis due to a Coelomycetous fungus: Case reports and review of the literatureCornea 2004 23:3-12. [Google Scholar]
[10]. Sutton DA, Coelomycetous fungi in human disease. A review: Clinical entities, pathogenesis, identification and therapyRev Iberoam Micol 1999 16:171-9. [Google Scholar]
[11]. Shukla P, Kumar M, Keshava G, Mycotic keratitis: an overview of diagnosis and therapyMycoses 2008 51:183-99. [Google Scholar]