Introduction: Coronary artery disease is the most common catastrophic disease in India. The safety and effectiveness of dual vessel sirolimus-eluting stent (SES) implantation (used as an intervention in CAD) is currently unknown in Indian population. The purpose of this study was to investigate one year clinical outcomes of patients with dual vessel coronary artery disease after implantation of the Supralimus-Core SES, in a "real-world" setting.
Materials and Methods: We evaluated 60 patients between April-2011 and August-2012, who underwent dual vessel percutaneous coronary intervention (PCI) with the Supralimus-Core SES implantation at the same index procedure. Dual vessels were defined as involvement of two major epicardial vessels (right, left anterior descending, circumflex, or left main coronary arteries) or one major epicardial vessel and a branch (≥2.5 mm in diameter) originating from another major epicardial vessel. The primary endpoint was target lesion failure (TLF) defined as the composite of cardiac death, myocardial infarction (MI), and clinically-driven target lesion revascularization (TLR) at one year. Secondary endpoint included combined (definite, probable and possible) stent thrombosis (ST).
Results: A total of 120 lesions were treated in 60 enrolled patients (mean age 56.0±9.2 y; 80.0% male) with average stent length of 23.1±8.5 mm. Among 60 patients, diabetes, hypertension and hypercholesterolemia were present in 15 (25.0%), 22 (36.7%) and 25 (41.7%) patients respectively. Indications for PCI were unstable angina in 30 (50.0%) patients and stable angina in 11 (18.3%) patients. Overall, 40 (33.3%) lesions were classified as complex (American College of Cardiology/American Heart Association type B2/C). The cumulative TLF rate was 5.0% (n=3) at one year. Cardiac death, MI and clinically-driven TLR occurred in 1 (1.7%), 0 (0%) and 2 (3.3%) patients, respectively at one year follow-up. The Kaplan-Meier curve of the freedom from overall events at one year was 95.0%. According to the Academic Research Consortium definition, there were no events of stent thrombosis during one year.
Conclusion: Our study shows that, dual vessel Supralimus-Core SES implantation allows safe and effective treatment with low rates of TLF at one year follow-up in Indian population.
Introduction
The safety and efficacy of percutaneous transluminal coronary angioplasty (PTCA) has been demonstrated in selected patients with symptomatic coronary artery disease [1,2] . Application of PTCA in patients with multiple vessels or in more extensive coronary artery disease has been limited, and the safety and short- and long-term efficacy are less clear [3-5] . Stent implantation has added an important dimension to percutaneous re-vascularization strategies and has been shown to be an effective rescue device after acute or threatened vessel closure after failed PTCA [6,7] .
However, some studies with multivessel disease reported higher restenosis and repeat revascularization rates in patients treated with bare metal stents (BMS) than in those after surgical treatment [8-12] . The introduction of drug-eluting stents (DES) indicating advantage over bare-metal stents in reducing the restenosis incidence and has narrowed the re-intervention gap between percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery in multivessel coronary artery disease (CAD) [8-10] [13] [16] . Additionally, performing multivessel PCI in a single index procedure has potential economic and social advantages [17] .
Here, we present our experience of the use of the Supralimus-Core (Sahajanand Medical Technologies Pvt. Ltd., Surat, India) sirolimus-eluting stent (SES) in patients with dual vessel CAD in an unselected real-world population. No study was especially designed to evaluate the safety and effectiveness of SES in patients with dual vessel disease. The main aim of the study was to conduct a multicenter, observational study including patients with dual vessel CAD, and treated solely with multiple Supralimus-Core SES implantations in a real-world setting, and to report the short (30 days), medium (6 month), and long-term (one year) clinical outcomes.
Materials and Methods
Study design and patient population
This was a multi-center, retrospective, observational study conducted at three investigational sites in India; which included 60 patients, treated between April-2011 and August-2012, with dual vessel stenting using the Supralimus-Core SES at the same index procedure. Patients included in this study had either stable or unstable angina or silent ischemia and underwent dual vessel stenting using the Supralimus-Core SES. Dual vessels were defined as involvement of two major epicardial vessels (right, left anterior descending, circumflex, or left main coronary arteries) or one major epicardial vessel and a branch (≥2.5 mm in diameter) originating from another major epicardial vessel. The study was conducted in accordance with the Declaration of Helsinki and study protocol was approved by the Institutional Review Board or Ethics Committee of each participating centres. All patients provided written informed consent prior to their inclusion in the study.
Endpoints of the study and definitions
The primary endpoint of the study was target lesion failure (TLF), defined as cardiac death, myocardial infarction (MI), or clinically-driven target lesion revascularization (TLR) by percutaneous or surgical methods at 1-year. Secondary endpoint included combined (definite, probable and possible) stent thrombosis (ST). All deaths were considered cardiac unless a clear non-cardiac cause could be established. The diagnosis of MI was based on either the development of new pathological Q-waves in ≥2 contiguous electrocardiogram leads and/or elevation of creatine kinase myocardial band isoenzyme level more than three times the upper normal limit after the procedure during index hospitalization, or cardiac enzyme level elevation more than two times the upper normal limit thereafter. TLR was defined as clinically-driven repeat PCI of the target lesion or CABG including the target vessel. Target vessel revascularization (TVR) was defined as clinically-driven repeat PCI or CABG of the target vessel. Stent thrombosis was classified according to the Academic Research Consortium (ARC) definition [18] .
Interventional procedure and medical therapy
Coronary angioplasty and the Supralimus-Core SES implantation were performed according to standard practice. Pre-dilatation or direct stenting were performed at the discretion of the operator. Antiplatelet therapy with 300 mg of clopidogrel was administered orally within 24 h before the procedure, unless the patient was already taking clopidogrel. At the start of procedure all patients received intra-arterial bolus of unfractionated heparin (50 to 150 IU/Kg) to achieve an activated clotting time between 250 and 300 sec. Glycoprotein IIb/IIIa inhibitors were used at the physician’s discretion. After the procedure aspirin was continued lifelong and clopidogrel administration was recommended for at least 12-months, although standard ischemic therapy was prescribed according to patients conditions at discharge.
Data collection and follow-up
Clinical follow-up was scheduled by telephone communication or office visit at 30 days, 6 month and one year after the index procedure; no patient was lost to follow-up. Follow-up information on all patients was obtained in a prospective manner. The data including demographic information, cardiovascular history, comorbidities, lesion and procedure characteristics, and antithrombotic regimens were collected. These centers provided hard paper copies that were subsequently entered into the database by the data management team. Documentation of adverse events which occurred at other institutions during the follow-up period was obtained from the local physicians and hospital records.
Statistical Analysis
Descriptive statistical analysis was performed by using continuous variables expressed as means with standard deviations and by using categorical variables presented as percent frequency. The cumulative survival rate free from events was calculated by the Kaplan-Meier analysis. All data were processed using the Statistical Package for Social Sciences, version 15 (SPSS, Chicago, IL, USA).
Results
Patient demographic, lesion and procedural characteristics
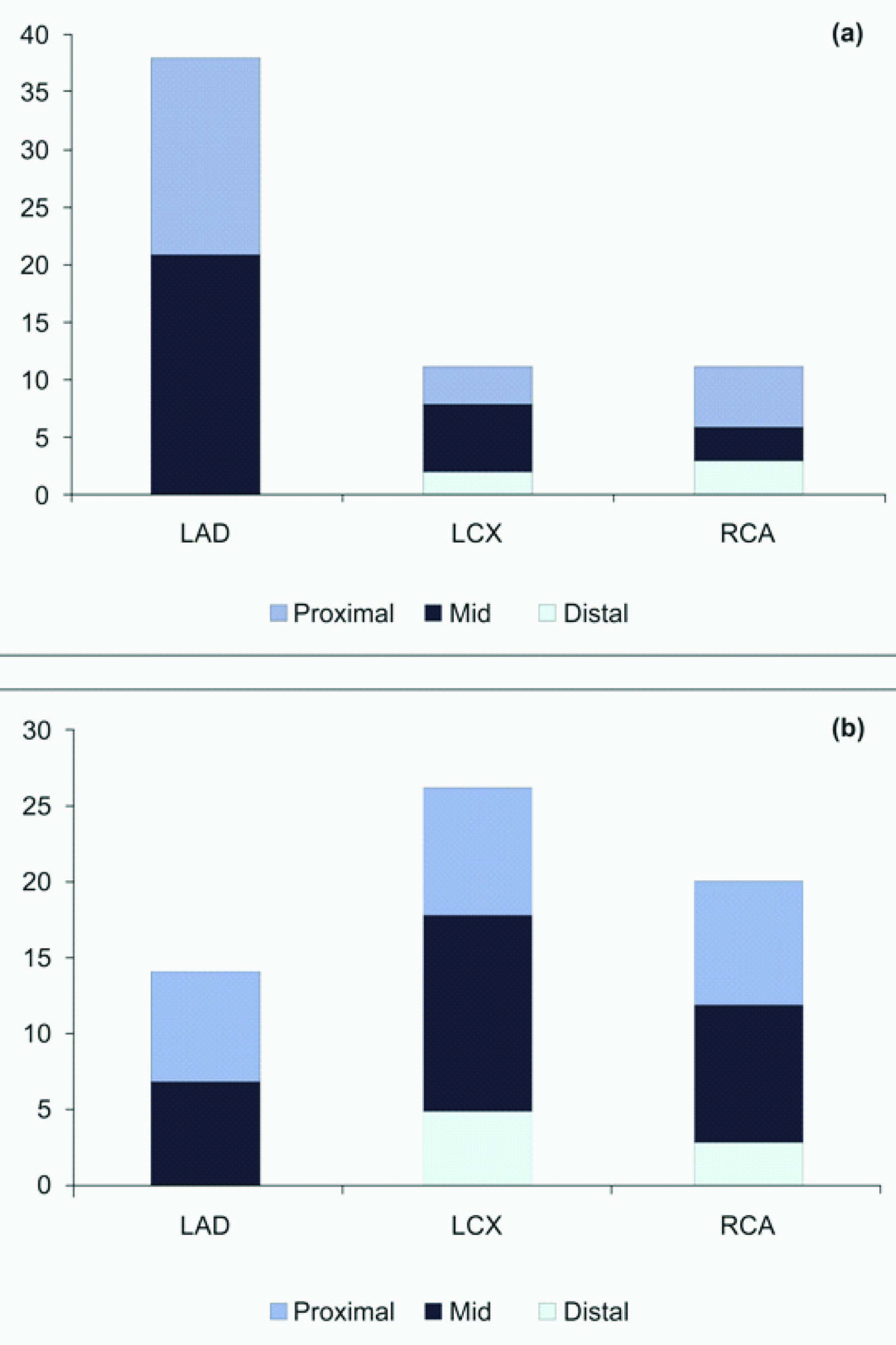
The study included 60 patients (mean age 56.0±9.2 y; 80.0% male) with dual vessel stenting using the Supralimus-Core SES. Patient demographic characteristics including risk factors for coronary artery disease are presented in [Table/Fig-1]. The co-morbidities i.e. diabetes mellitus, hypertension and hypercholesterolemia were present in 15 (25.0%), 22 (36.7%) and 25 (41.7%) patients respectively. Previous MI had occurred in 8 (13.3%) patients and indications for PCI were unstable and stable angina in 30 (50.0%) and 11 (18.3%) patients, respectively. The lesion and procedural characteristics are listed in [Table/Fig-2,Table/Fig-3]. Most of the target lesions were located in the left anterior descending artery 52 (43.3%) predominantly in proximal or mid-segments of the treated vessel (89.2%). Overall, 40 (33.3%) lesions were classified as complex (American College of Cardiology/American Heart Association type B2/C), including chronic total occlusions in 8 (6.7%) patients. A total of 125 stents were implanted at index procedure (2.1 stents per patient) with an average diameter and total stent length of 3.0±0.3 mm and 23.1±8.5 mm, respectively.
Cumulative clinical outcomes
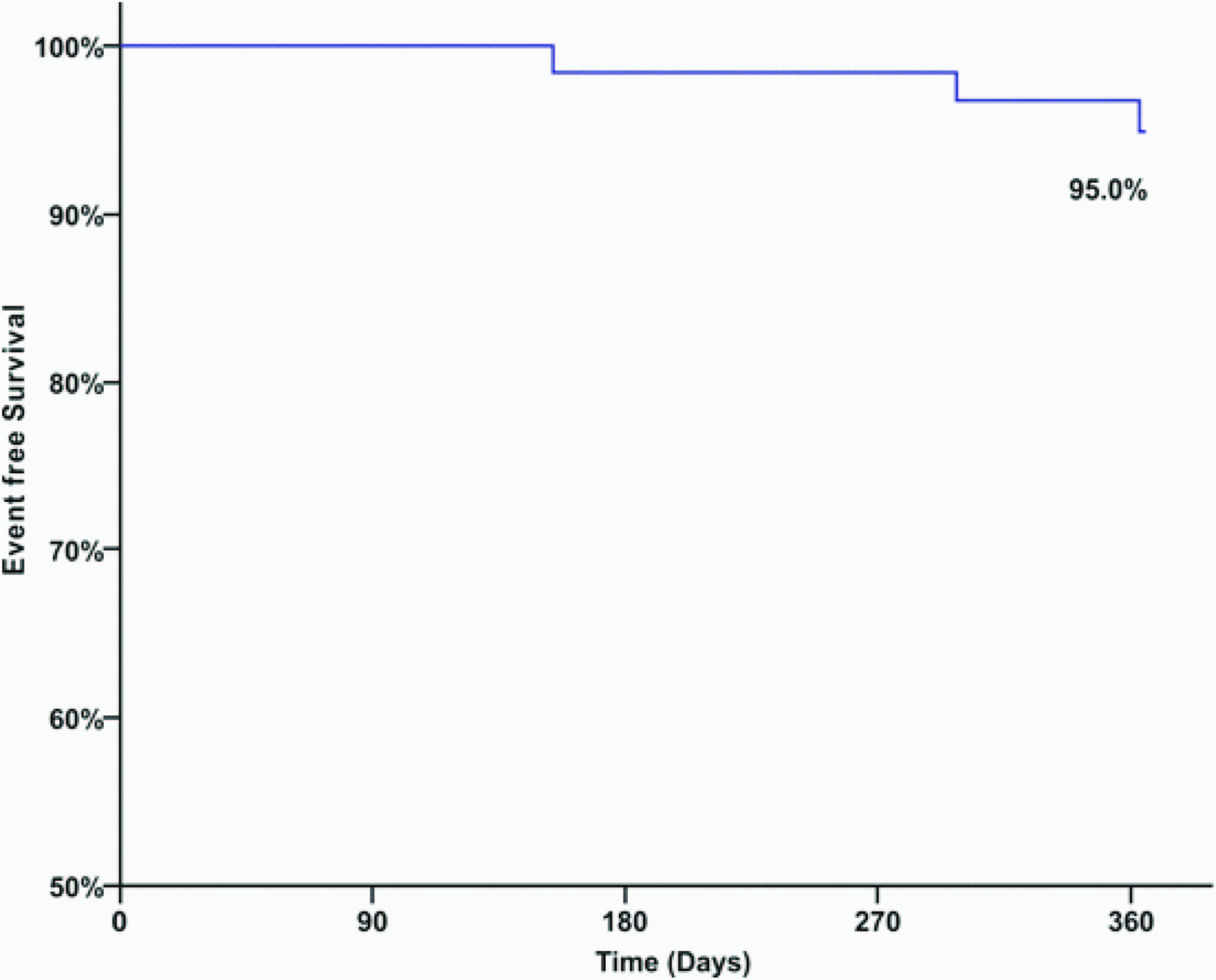
The 30 day, 6 month and one year clinical outcomes for the overall study population are shown in [Table/Fig-4]. The primary endpoint, a cumulative TLF at one year follow-up, occurred in 3 (5.0%) of 60 patients, consisting of 1 (1.7%) cardiac deaths, 0 (0%) myocardial infarction and 2 (3.3%) clinically-driven TLR. One patient died at ten months after the index procedure because of progressive congestive heart failure. The one year event-free survival curve of the study patients is depicted in [Table/Fig-5]. At 1-year, the cumulative composite incidence of ARC definite and probable stent thrombosis, the main secondary endpoint of the study, was 0 %.
Discussion
Restenosis which is known to be secondary to intimal hyperplasia [19] is the main drawback of PCI and this remains a major problem which is responsible for the majority of repeat revascularization procedures. The repeat revascularization rate in patients undergoing multivessel PTCA is higher than that observed in the treatment of single vessel [20] . Moreover, after PTCA, severe dissection is the most common cause of abrupt vessel closure. For example, abrupt vessel closure occurred in 9.5% of PTCA cases in the BARI (Bypass Angioplasty Revascularization Investigation) trial [21] compared with the approximately 4% rate of abrupt vessel closure for single-lesion PTCA [22] .
Various trials have demonstrated that multivessel PTCA is associated with a greater incidence of recurrent angina necessitating another revascularization procedure compared to CABG [23,24] . After failed angioplasty, stents have been demonstrated to be an effective bailout device to reduce the need for emergency or urgent CABG [6,25] . Now the question arises as to whether the reduction in stent restenosis seen with DES in simple lesions [26,27] can be extended to their use in multivessel disease. Laham et al., [28] reported the results of multivessel stenting in 103 patients and mortality; Q-wave and non–Q-wave MI rates were 1%, 2% and 11%, respectively. Importantly, no patients required emergent CABG at long-term follow-up and event-free survival was 79%. Moussa et al., [29] reported the results of 100 patients undergoing multivessel coronary stenting and during follow up, the mortality, CABG and TVR rates were 4%, 2% and 30%, respectively. Also in our study, no patients required emergent CABG at one year follow-up. These results altogether suggest that multivessel stenting in appropriately selected patients may be a viable therapeutic strategy for patients with multivessel coronary disease.
The Supralimus-Core uses L605 Co-Cr alloy (60 μm strut thickness) as its stent platform which is coated with a biodegradable polymer to deliver sirolimus and, its clinical safety and effectiveness was already demonstrated in various clinical studies [30-34] . In MAXIMUS study, the Supralimus-Core SES has proved its effectiveness by reducing restenosis at 8-months and safety with an acceptable rate of cardiac events at 12-months [30] . Recent, Supralimus-Core optical coherence tomography (OCT) study showed better strut coverage (97.21% at 4-months) with functional endothelium, to avoid in-stent restenosis and to minimize risk of stent thrombosis [33] . Also, S-CORE multi-center registry conducted clearly provides evidence for the safe and effective use of the Supralimus-Core SES in unselected real-world population [34] .
In our study, all patients received dual vessel treatment with the Supralimus-Core SES and included a relatively high proportion of complex lesions and the American College of Cardiology/ the American Historical Association (ACC/AHA) types B2 and C lesions, which were less likely to be approached successfully in the randomized trials of multivessel disease. Despite of this, repeat revascularization (3.3%) in the current study at one year was comparable with rates reported in other series of percutaneous management of multivessel disease [35,36] . So, the use of the Supralimus-Core SES in this study did not cause a considerable increase in the rate of repeat revascularization with percutaneous treatment rather than CABG [37] .
The present study describes one year clinical outcomes of patients with dual vessel coronary artery disease after implantation of the Supralimus-Core SES, in a "real-world" setting. In this study, we demonstrated that TLF (cardiac death, MI, and clinically-driven TLR) occurred in 5.0% (n=3) of all patients, with cardiac death occurring in 1.7% (n=1) of patients with dual vessel disease at one year follow-up. Despite the high proportion of patients with high-risk characteristics and complex lesions, there were no events of stent thrombosis during one year. Kaplan-Meier analysis in the current study suggested that 95.0% of patients did not need subsequent hospitalization for heart disease during the first year of follow-up study.
Legalknowledgebasedonnursingqualification
| Characteristics | n = 60 Patients |
|---|
| Age (mean ± SD, yrs) | 56.0 ± 9.2 |
| Male, n (%) | 48 (80.0%) |
| Diabetes Mellitus, n (%) | 15 (25.0%) |
| Hypertension, n (%) | 22 (36.7%) |
| Smoker, n (%) | 11 (18.3%) |
| Hypercholesterolemia, n (%) | 25 (41.7%) |
| Family history of CAD, n (%) | 11 (18.3%) |
| PCI Indication |
| Stable Angina, n (%) | 11 (18.3%) |
| Unstable Angina, n (%) | 30 (50.0%) |
| Previous MI, n (%) | 8 (13.3%) |
| Previous PCI, n (%) | 7 (11.7%) |
| Previous CABG, n (%) | 1 (1.7%) |
| Previous Stroke, n (%) | 1 (1.7%) |
Lesion and procedural characteristics
LAD=left anterior descending; RCA= right coronary artery; LCX=left circumflex; ACC/AHA=the American College of Cardiology/ the American Historical Association
| Characteristics | Patients = 60 / Lesions = 120 |
|---|
| Target vessels |
| Left anterior descending artery, n (%) | 52 (43.3%) |
| Right coronary artery, n (%) | 31 (25.8%) |
| Left circumflex artery, n (%) | 37 (30.8%) |
| Location in vessels |
| Proximal, n (%) | 48 (40.0%) |
| Mid, n (%) | 59 (49.2%) |
| Distal, n (%) | 13 (10.8%) |
| Coronary vessels combination in dual vessel angioplasty |
| LAD + LCX, n (%) | 58 (48.3%) |
| LAD + RCA, n (%) | 46 (38.3%) |
| RCA + LCX, n (%) | 16 (13.3%) |
| ACC/AHA Lesion Classification |
| A, n (%) | 37 (30.8%) |
| B1, n (%) | 43 (35.8%) |
| B2, n (%) | 37 (30.8%) |
| C, n (%) | 3 (2.5%) |
| Total no. of stent, n | 125 |
| No. of stents per patient, (mean ± SD, mm) | 2.1 ± 0.3 |
| No. of stents per lesion, (mean ± SD, mm) | 1.0 ± 0.2 |
| Average Stent Length, (mean ± SD, mm) | 23.1 ± 8.5 |
| Average Stent Diameter, (mean ± SD, mm) | 3.0 ± 0.3 |
| Total occlusion, n (%) | 8 (6.7%) |
Lesion Localization (a) lesion-1 and (b) lesion-2
Cumulative clinical outcomes during 30-days, 6-month and 1-year of follow-up (n=60)
CABG=coronary artery bypass graft; TLR=target lesion revascularization; TVR=target vessel revascularization
| 30-days | 6-months | 12-months |
|---|
| Target lesion failure, n (%) | 0 (0%) | 1 (1.7%) | 3 (5.0%) |
| Death, n (%) | 0 (0%) | 0 (0%) | 1 (1.7%) |
| Cardiac Death, n (%) | 0 (0%) | 0 (0%) | 1 (1.7%) |
| Non-cardiac Death, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Myocardial Infarction, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CABG, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Clinically-driven TLR, n (%) | 0 (0%) | 1 (1.7%) | 2 (3.3%) |
| Clinically-driven TVR, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Stent thrombosis, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
Kaplan-Meier curve of cumulative event-free survival over 1-year
Study Limitations
There are several limitations to our study. First, this is a retrospective, observational study rather than a prospective randomized clinical trial designed to assess the safety and efficacy of stents in patients with dual vessel disease. Second, there are data of selected patients who underwent a successful procedure with only Supralimus-Core SES at same index procedure. Third, longer term (>1-year) follow up in a larger cohort of patients is necessary in order to assess the true rate of restenosis.
Conclusion
Our study shows that, dual vessel Supralimus-Core SES implantation allows safe and effective treatment with low rates of TLF at one year follow-up in Indian population. Despite the complex lesion morphology, there were no events of stent thrombosis during 1-year. Longer follow-up in a large population is clearly required.
Disclosure
Dr. Ashok Thakkar and Mr. Bhavesh Khambhati, are the employees of Sahajanand Medical Technologies Pvt. Ltd. and have provided detailed assistance in literature search and manuscript writing. Other authors declare that they have no conflict of interest. No grant was received from Sahajanand Medical Technologies Pvt. Ltd. for the study.
[1]. KM Kent, LG Bentivoglio, PC Block, MG Bourassa, MJ Cowley, G Dorros, Long-term efficacy of percutaneous transluminal coronary angioplasty (PTCA): report from the National Heart, Lung, and Blood Institute PTCA RegistryAm J Cardiol 1984 53(12):27C-31C. [Google Scholar]
[2]. MJ Cowley, G Dorros, SF Kelsey, KM Van Raden, M Detre, Acute coronary events associated with percutaneous transluminal coronary angioplastyAm J Cardiol 1984 53(12):12C-6C. [Google Scholar]
[3]. G Dorros, SH Stertzer, MJ Cowley, RK Myler, Complex coronary angioplasty: multiple coronary dilatationsAm J Cardiol 1984 53(12):126C-30C. [Google Scholar]
[4]. G Dorros, RF Lewin, L Janke, Multiple lesion transluminal coronary angioplasty in single and multivessel coronary artery disease: acute outcome and long-term effectJ Am Coll Cardiol 1987 10(5):1007-13. [Google Scholar]
[5]. G Dorros, RF Lewin, LM Mathiak, Complex angioplasty: single versus multiple dilatations in multivessel coronary disease patientsCardiol Clin 1989 7(4):783-89. [Google Scholar]
[6]. BS George, WD Voorhees, GS Roubin, NE Fearnot, CA Pinkerton, AE Raizner, Multicenter investigation of coronary stenting to treat acute or threatened closure after percutaneous transluminal coronary angioplasty: clinical and angiographic outcomesJ Am Coll Cardiol 1993 22(1):135-43. [Google Scholar]
[7]. JJ Goy, U Sigwart, P Vogt, JC Stauffer, L Kappenberger, Long-term clinical and angiographic follow-up of patients treated with the self-expanding coronary stent for acute occlusion during balloon angioplasty of the right coronary arteryJ Am Coll Cardiol 1992 19(7):1593-96. [Google Scholar]
[8]. PW Serruys, F Unger, JE Sousa, A Jatene, HJ Bonnier, JP Schonberger, Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel diseaseN Engl J Med 2001 344(15):1117-24. [Google Scholar]
[9]. SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trialLancet 2002 360(9338):965-70. [Google Scholar]
[10]. A Rodriguez, V Bernardi, J Navia, J Baldi, L Grinfeld, J Martinez, Argentine Randomized Study: Coronary Angioplasty with Stenting versus Coronary Bypass Surgery in patients with Multiple-Vessel Disease (ERACI II): 30-day and one-year follow-up results. ERACI II InvestigatorsJ Am Coll Cardiol 2001 37(1):51-58. [Google Scholar]
[11]. R Kornowski, R Mehran, LF Satler, AD Pichard, KM Kent, A Greenberg, Procedural results and late clinical outcomes following multivessel coronary stentingJ Am Coll Cardiol 1999 33(2):420-26. [Google Scholar]
[12]. I Moussa, B Reimers, J Moses, C Di Mario, L Di Francesco, M Ferraro, Long-term angiographic and clinical outcome of patients undergoing multivessel coronary stentingCirculation 1997 96(11):3873-79. [Google Scholar]
[13]. SN Hoffman, JA TenBrook, MP Wolf, SG Pauker, DN Salem, JB Wong, A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: one- to eight-year outcomesJ Am Coll Cardiol 2003 41(8):1293-304. [Google Scholar]
[14]. VM Legrand, PW Serruys, F Unger, BA van Hout, MC Vrolix, GM Fransen, Three-year outcome after coronary stenting versus bypass surgery for the treatment of multivessel diseaseCirculation 2004 109(9):1114-20. [Google Scholar]
[15]. D Orlic, E Bonizzoni, G Stankovic, F Airoldi, A Chieffo, N Corvaja, Treatment of multivessel coronary artery disease with sirolimus-eluting stent implantation: immediate and mid-term resultsJ Am Coll Cardiol 2004 43(7):1154-60. [Google Scholar]
[16]. GW Stone, SG Ellis, DA Cox, J Hermiller, C O'Shaughnessy, JT Mann, A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease N Engl J Med 2004 350(3):221-31. [Google Scholar]
[17]. F Cuculi, AP Banning, A Abizaid, AL Bartorelli, AC Baux, V Dzavik, Outcomes in patients undergoing multivessel percutaneous coronary intervention using sirolimus-eluting stents: a report from the e-SELECT registryEuroIntervention 2011 7(8):962-68. [Google Scholar]
[18]. DE Cutlip, S Windecker, R Mehran, A Boam, DJ Cohen, GA van Es, Clinical end points in coronary stent trials: a case for standardized definitionsCirculation 2007 115(17):2344-51. [Google Scholar]
[19]. R Hoffmann, GS Mintz, GR Dussaillant, JJ Popma, AD Pichard, LF Satler, Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound studyCirculation 1996 94(6):1247-54. [Google Scholar]
[20]. L Lambert, L Bonan, L Cote, L Crepeau, L de Guise, PJ Lesperance, Early results, complications and restenosis rates after multilesion and multivessel percutaneous transluminal coronary angioplastyAm J Cardiol 1987 60(10):788-91. [Google Scholar]
[21]. The BARI Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease . The Bypass Angioplasty Revascularization Investigation (BARI) InvestigatorsN Engl J Med 1996 335(4):217-25. [Google Scholar]
[22]. JA Bittl, Advances in coronary angioplastyN Engl J Med 1996 335(17):1290-302. [Google Scholar]
[23]. A Rodriguez, E Mele, E Peyregne, F Bullon, N Perez-Balino, MI Liprandi, Three-year follow-up of the Argentine Randomized Trial of Percutaneous Transluminal Coronary Angioplasty Versus Coronary Artery Bypass Surgery in Multivessel Disease (ERACI)J Am Coll Cardiol 1996 27(5):1178-84. [Google Scholar]
[24]. CW Hamm, J Reimers, T Ischinger, HJ Rupprecht, J Berger, W Bleifeld, A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI) N Engl J Med 1994 331(16):1037-43. [Google Scholar]
[25]. V Mathew, D Hasdai, DR Holmes, KN Garratt, MR Bell, A Lerman, Clinical outcome of patients undergoing endoluminal coronary artery reconstruction with three or more stentsJ Am Coll Cardiol 1997 30(3):676-81. [Google Scholar]
[26]. JW Moses, MB Leon, JJ Popma, PJ Fitzgerald, DR Holmes, C O'Shaughnessy, Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary arteryN Engl J Med 2003 349(14):1315-23. [Google Scholar]
[27]. A Colombo, J Drzewiecki, A Banning, E Grube, K Hauptmann, S Silber, Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesionsCirculation 2003 108(7):788-94. [Google Scholar]
[28]. RJ Laham, KK Ho, DS Baim, RE Kuntz, DJ Cohen, JP Carrozza, Multivessel Jr, Palmaz-Schatz stenting: early results and one-year outcomeJ Am Coll Cardiol 1997 30(1):180-85. [Google Scholar]
[29]. I Moussa, B Reimers, J Moses, C Di Mario, L Di Francesco, M Ferraro, Long-term angiographic and clinical outcome of patients undergoing multivessel coronary stentingCirculation 1997 96(11):3873-79. [Google Scholar]
[30]. A Seth, P Chandra, NS Chouhan, AS Thakkar, A first-in-man study of sirolimus-eluting, biodegradable polymer coated cobalt chromium stent in real life patientsIndian Heart J 2012 64(6):547-52. [Google Scholar]
[31]. AS Thakkar, AD Abhyankar, SI Dani, DN Banker, PI Singh, SA Parmar, Systemic exposure of sirolimus after coronary stent implantation in patients with de novo coronary lesions: Supralimus-Core(R) pharmacokinetic studyIndian Heart J 2012 64(3):273-79. [Google Scholar]
[32]. AD Abhyankar, AS Thakkar, In vivo assessment of stent recoil of biodegradable polymer-coated cobalt-chromium sirolimus-eluting coronary stent systemIndian Heart J 2012 64(6):541-46. [Google Scholar]
[33]. A Abhyankar, J Prajapati, S Reddy, Early vascular healing with biodegradable polymer coated sirolimus-eluting coronary stent implantation: assessed by optical coherence tomography results at 4-month follow-upMinerva Cardioangiol 2013 61(3):313-22. [Google Scholar]
[34]. P Chandwani, AD Abhyankar, JS Prajapati, SC Porwal, AS Thakkar, Clinical Performance of the Cobalt-Chromium Biodegradable Polymer Coated Sirolimus-Eluting Stent in an Unselected Real-World PopulationInt J Clin Med 2014 5(5):206-15. [Google Scholar]
[35]. MR Bell, KR Bailey, GS Reeder, AC Lapeyre, DR Holmes, Percutaneous transluminal angioplasty in patients with multivessel coronary disease: how important is complete revascularization for cardiac event-free survival?J Am Coll Cardiol 1990 16(3):553-62. [Google Scholar]
[36]. D Hasdai, PB Berger, MR Bell, CS Rihal, KN Garratt, DR Holmes, The changing face of coronary interventional practice. The Mayo Clinic experienceArch Intern Med 1997 157(6):677-82. [Google Scholar]
[37]. DP Faxon, K Ghalilli, AK Jacobs, NA Ruocco, EM Christellis, MA Kellett, The degree of revascularization and outcome after multivessel coronary angioplastyAm Heart J 1992 123(4 Pt 1):854-59. [Google Scholar]