Introduction
Type 2 Diabetes Mellitus (T2DM) presents a mounting menace to health and according to recent forecast will become even bigger concern. WHO estimates that in 2030, Diabetes will be the 7th leading cause of death [1]. Development of new strategies for the prevention and treatment of Type 2 Diabetes Mellitus is a scientific test of high concern.
Ghrelin, also known as the ‘hunger hormone is the only identified peptide hormone that is produced by the peripheral organs and acts on the brain to stimulate the appetite. It is a multifaceted 28-amino acid peptide hormone secreted by endocrine X/A-like cells of the stomach mucosa, intestinal mucosa, the arcuate nucleus of the hypothalamus, the pituitary, and other tissues. It is also produced in the pancreatic islets where it acts as an autocrine/ paracrine growth factor [2]. It was originally discovered by Kojima in 1999 [3] as the natural ligand of the GH secretagogue receptor type 1a (GHS-R1a) and thus a potent GH-stimulating factor [3-5]. Subsequently, researches have demonstrated that Ghrelin has a wide spectrum of other biological activities, like stimulation of secretion of hormones like ACTH and prolactin, stimulation of gastric motility, stimulation of gastric acid secretion and stimulation of appetite to mention a few [6,7]. Ghrelin signaling also plays a vital role in influencing cardio protection, bone metabolism and muscle atrophy [8-11]. These versatile biological roles of Ghrelin have opened many new research avenues and have made Ghrelin a highly attractive target for the discovery of new drugs. With ghrelin playing roles in various physiological processes, the ghrelin-GOAT system presents an attractive therapeutic target.
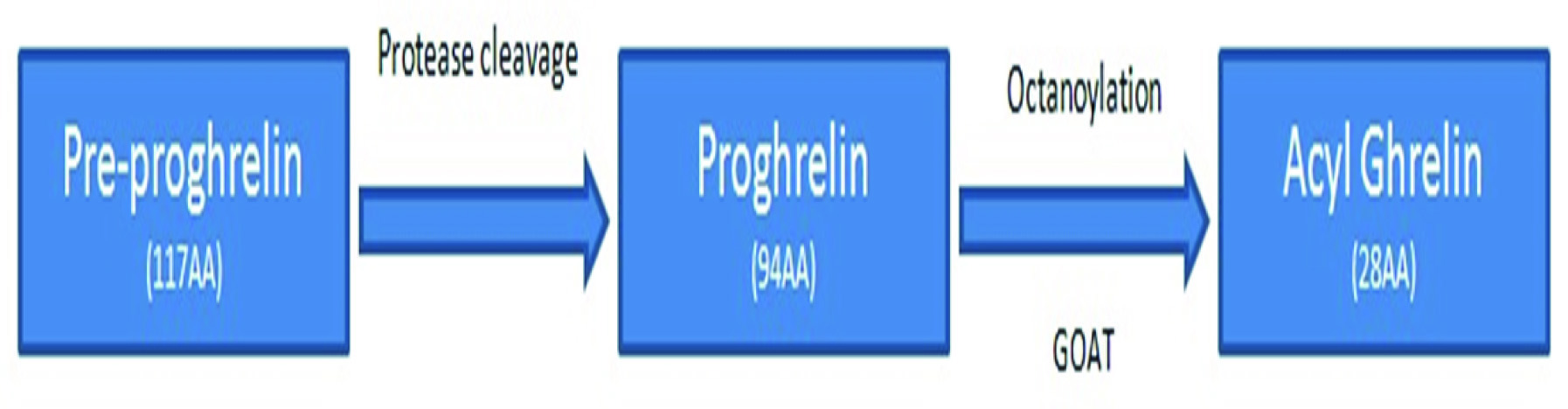
Ghrelin is cleaved post-translationally by furin-like proteases from a 117 amino acid, preproghrelin [12]. Following cleavage, enzyme ghrelin O-acyltransferase (GOAT) can octanoylate proghrelin to form acylated ghrelin [13] [Table/Fig-1]. GOAT is the only identified enzyme till date that particularly modifies the third amino acid serine mellitusin ghrelin and catalyzes the acyl modification of ghrelin. It prefers n-hexanoyl-CoA as the acyl donor over n-octanoyl-CoA. It modifies a four-amino acid peptide obtained from the N-terminal of ghrelin, which indicates that these amino acids form the main motif for substrate identification by GOAT [14]. The acylation of ghrelin by enzyme ghrelin-O-acyltransferase (GOAT) is essential for binding to its receptor. Acytylated ghrelin may then exert a stimulating effect on stimulation and play a significant role in modulating metabolism via a variety of mechanisms [15]. However, the exact mechanism of action and influence on energy balance and glucose metabolism of GOAT is yet to be explored. The discovery of GOAT arouses many significant queries about several possible therapeutic interventions for the treatment of obesity, T2DM and other metabolic disorders.
The current literature provides limited information regarding use of GOAT for the treatment of obesity, T2DM and other metabolic disorders. The prospective therapeutic advantages of using ghrelin–GOAT system in countering obesity and diabetes are striking but yet fully unexplored. This review elaborates the new researches describing the role of GOAT in regulating energy balance and glucose metabolism. Information in this review will guide researchers and professionals in considering use of GOAT for the same.
Objectives of the study: To review the biological role of GOAT in the regulation of energy balance and glucose metabolism and explore the probable therapeutic avenues of GOAT for the treatment of obesity, T2DM and other metabolic disorders.
Methods
Types of studies: Controlled trials (RCT) of the parallel or crossover design, reviews and books which evaluated the effects of ghrelin or GOAT on energy and glucose metabolism were searched. Studies conducted in both hospital and community settings from 1999 to 2014 were eligible for inclusion in the review. Studies published in non-peer reviewed journals or only in abstract forms were not included in the review.
Search Methods for Identification of Studies
Electronic searches: All publications describing effect of ghrelin or GOAT on energy and metabolic control were sought through electronic searches on the PUBMED, Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library, MEDLINE (1999 to June 2013), EMBASE (1999 to June 2013), CINAHL (1999 to June 2013) and Digital Dissertations. Studies were also searched in the Database of Abstracts of Reviews of Effects (DARE), NHS Centre for Reviews and Dissemination (CRD) databases. The bibliographies of all papers were searched for further studies. No restrictions regarding the language of publication were imposed. To avoid missing of studies in the search strategy, consideration was given to the spelling of terms used in different countries. If any data was found insufficient, the authors were contacted through e-mail. Different terms were used for different databases. For Pubmed search builder used was (“Ghrelin/physiology”{Mesh}) AND (“Metab*{Mesh} OR “Carbohydrate Metab*”{Mesh} OR “Lipid Metab*”{Mesh} OR “Energy Metab*”{Mesh} OR “metab*” {Subheading} OR “Glucose Metabolism Disorders”{Mesh} OR “insulin” OR “Diab*”{Mesh}
Searching other resources: Book chapters and editorials were scanned and hand searches were conducted for journals, and conference proceedings. Experts and authors on the subject were contacted through emails for potential missing and unpublished studies and asked to contribute published and unpublished material.
Ghrelin in Glucose Metabolism
Several studies have demonstrated that Ghrelin is a key regulator of energy balance and glucose metabolism [16,19]. It acts as an important endocrine link connecting various biological processes concerned in the regulation of food intake, energy balance and glucose metabolism [20]. Ghrelin has novel actions on the handling of key components of glucose metabolism by regulating gluconeogenesis/ glycogenolysis and by inhibiting insulin secretion and insulin sensitivity [9,21]. However, its mechanism of action and influence on whole glucose metabolism remains unexplored.
Role of Ghrelin Isoforms in Glucose Metabolism
Endogenous ghrelin exists in two forms, as a pure unacylated 28-amino acid peptide (desacyl ghrelin/ dAG/ unacytylated/ UAG) and as an acylated peptide (acyl ghrelin/ AG) with a fatty acid side chain [22].The ratio of AG/ UAG is particularly important in terms of glucose metabolism. Studies have shown that the metabolic status alters the ratio of circulating AG to UAG [20] and that majority of ghrelin circulates in the unacylated form which was considered to be devoid of any endocrine action [23]. Studies have reported that the physiological effects of ghrelin are carried out by the acylated form (AG) and not by unacytylated form (UAG). However; in other effects UAG can imitate the actions of ghrelin or even exert opposite effects [24]. Reports have suggested that the action of UAG in the brain plays a role in the regulation of appetite [25,26]. In mice and humans, UAG was shown to either have no effect on glucose metabolism [22,27], or to have positive effects on sensitivity [28] and secretion of insulin [29,31]. Some studies have suggested that desacyl ghrelin improves insulin sensitivity [27] decreases hepatic glucose output [32] and thereby opposes the acyl ghrelin-mediated systemic regulation of glucose metabolism [33].
The acylated form of Ghrelin is a multifunctional peptide hormone that plays a role in insulin release, appetite, gut motility, and glucose metabolism. It also has plays role in the regulation of growth, cardiovascular and reproductive systems [34]. Studies in rodents and humans have shown that systemic infusion of AG elevates blood glucose, inhibits insulin secretion [25,34], increases food intake and adiposity [35] and thereby impairs glucose metabolism [32]. Centrally, AG is believed to promote a positive energy balance and peripherally, regulates insulin secretion by acting on islet cells of the pancreas [13]. Conflicting results are available about the effects of both ghrelin isoforms on glucose metabolism [36].
Goat
The discovery of Ghrelin O-acyltransferase (GOAT) seems to be an important step forward in the understanding of ghrelin processing. Much attention is being focused on the GOAT which is emerging as a potential diagnostic and therapeutic target for regulating appetite and conditions related to the ghrelin axis, such as obesity, insulin resistance and T2DM [37].
GOAT was identified in 2008 [20,38] as a member of membrane Bound O-Acyl Transferase (MBOAT) family, a group of integral membrane proteins involved in lipid-biosynthetic and lipid-signaling reactions. MBOAT family members are composed of many multispanning membrane proteins from prokaryotes and eukaryotes, including 11 in humans and have diverse substrates like proteins, phospholipids and sterols. They have been shown to be critical for lipid biosynthesis, sterol acylation, and acyl modification of secreted proteins, including ghrelin. However, the enzymatic properties of this family as integral membrane proteins is yet unexplored. Till date, ghrelin is the only known octanoylated protein and the only substrate for GOAT.
The structural domains is highly conserved among species. Very high similarity in GOAT proteins was found from humans to zebrafish [36] and it is interesting to note that GOAT of zebrafish, rat, and mouse have the capacity to acylate human ghrelin [13]. GOAT, like ghrelin, is produced by the stomach, and also in tissues like pancreatic islets, prostate [2,39] and in breast cancer [40]. It is present in human plasma [8] and released into pancreatic microcirculations [41].
Expression of GOAT
In human tissues, high levels of GOAT transcripts are found in stomach and pancreas, and low levels in other tissues like other parts of gastrointestinal tract, gallbladder, liver, adrenal cortex, pituitary, thyroid, breast, ovary, placenta, prostate, testis, fallopian tube, lymph node, kidney, lung, muscle, myocardium, spleen [13,39]. The expression of GOAT is 5-fold greater within ghrelin cells and in major Ghrelin-secreting tissue like stomach as compared to the hypothalamus [40]. It is also high in pituitary where ghrelin has autocrine and paracrine effects [42]. This expression of GOAT is similar to that of Ghrelin and support that both endocrine and paracrine effect of locally produced AG is essential [39].
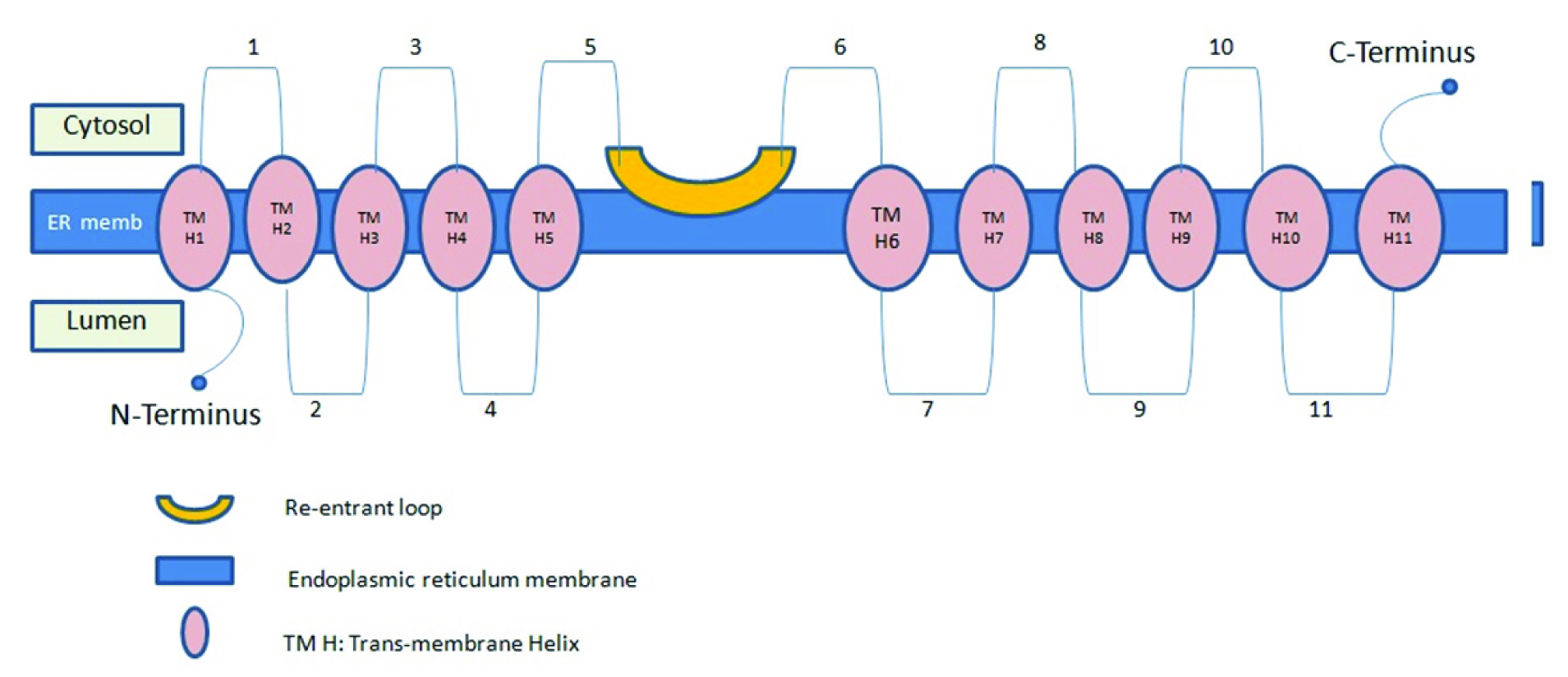
Structure of GOAT: Molecular mass for GOAT in its FC-16 micelle is 140.7 kDa. This size does not allow clear determination of whether purified GOAT is a monomer or dimer, although it is closer to a monomer position. GOAT is a polytopic integral membrane protein and contains 11 transmembrane helices (H1 to H11) and one reentrant loop but this needs further investigations [37] [Table/Fig-2].
The C-terminus of GOAT is located on the cytosolic side and N- terminus on the luminal side of the endoplasmic reticulum. The N- termini and C- termini are found on the opposite sides of the membrane, at first and eleventh TMs. Positions1,3,5,6,8 and 10 are cytosolic while the others are luminal. However; c6 does not cross the membrane and represents a reentrant loop [37] [Table/Fig-2].
Mechanism and site of action of GOAT
The action of GOAT has been studied on intact cells and membranes from insect cells that expresses mouse GOAT. GOAT uses a ternary complex mechanism of action.
The pre-prohormone (117-amino acid) is co-translationally cleaved to release proghrelin (94-amino acid) into the lumen of the endoplasmic reticulum (ER) where octanoate group is attached to serine-3 of proghrelin with the help of ghrelin O-acyltransferase (GOAT). GOAT recognizes several amino acids like glycine-1, serine-3 and phenylalanine-4 in preproghrelin that surrounds the site of octonoylation at serine 3. In a cell, the active site of GOAT must face the luminal side of the endoplasmic reticulum. However; to acylate proghrelin in vitro, atleast one fraction of GOAT protein must have their active sites facing the extraluminal side of the membrane [43]. The site of binding of proghrelin and the octanoyl-donor is yet not ascertained. So also; the identity of the Octanoyl donor and the active site is yet to be proved [44]. The C-terminus of acyl-proghrelin is then cleaved by prohormone convertase in the secretory granules to produce mature acyl-ghrelin [45].
Since GOAT is required for activation of Ghrelin, this enzyme is a good target for regulation of Ghrelin [42]. Thus GOAT is a critical component in synthesizing the bioactive form of Ghrelin, and thus plays a role in mediating the physiological functions of Ghrelin [36].
Regulation of GOAT mRNA Expression
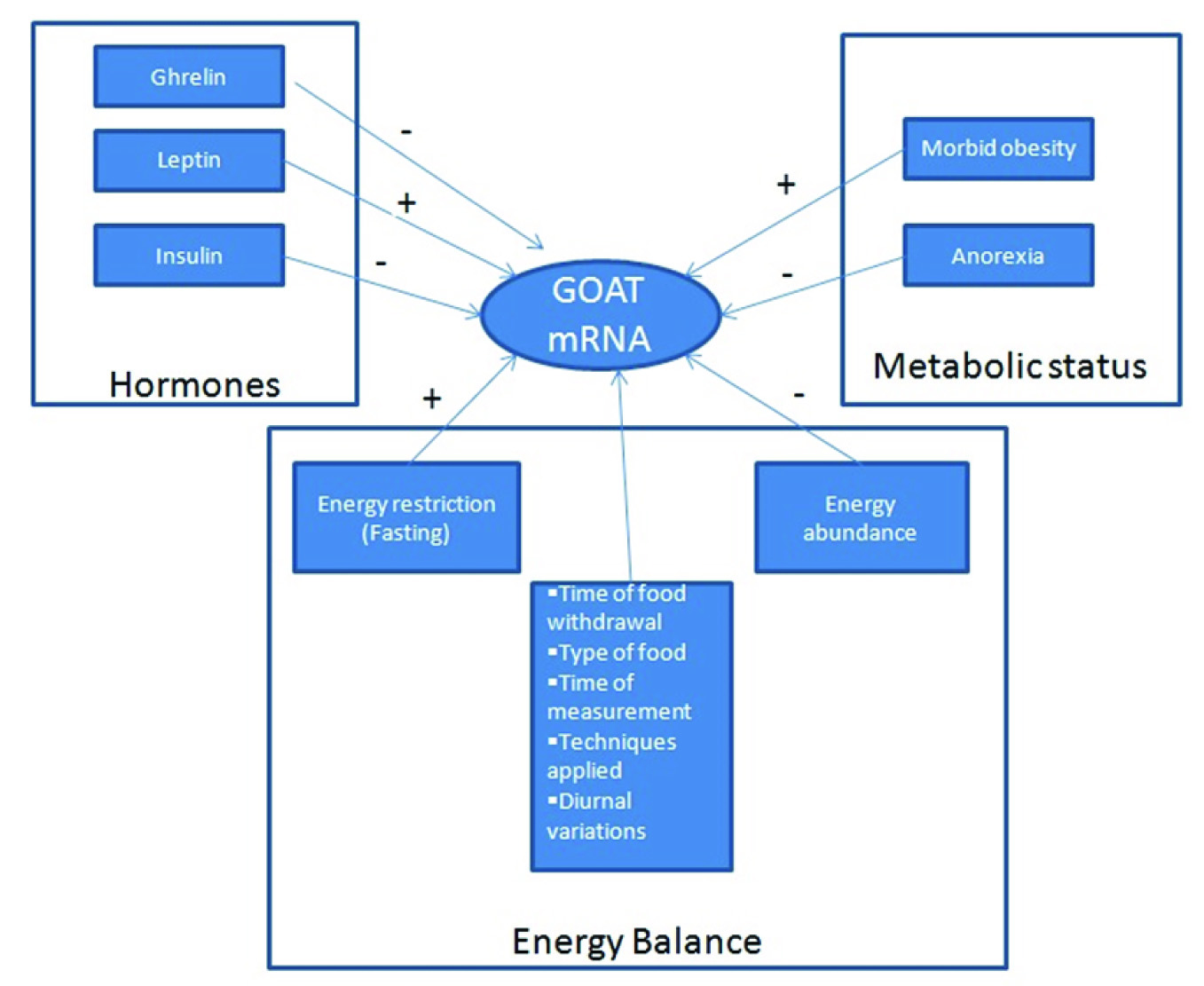
The expression of GOAT is encoded by the membrane bound O-acyl transferase (gene MBOAT4) [13,39]. It is regulated by energy balance, metabolic status and hormones such as leptin [36], somatostatin and by ghrelin itself. Unacytylated ghrelin does not seem to have a role in the regulation of the expression of GOAT [40].
Energy balance Metabolic Status
One of the prime regulators of GOAT mRNA expression is energy balance; the expression being up-regulated by energy restriction and down-regulated by energy abundance [20,40] [Table/Fig-3]. Fasting increases stomach GOAT mRNA levels [38]. However, Gonzalez et al., showed that GOAT mRNA levels are comparatively stable during fasting and once there is a marked weight loss in animals, the levels are strikingly increased [46] Kirchner’s study showed that fasting (12h, 24h and 36h) suppressed stomach GOAT mRNA in male mice [20]. These differences in expression of GOAT in fasting in the two studies may be related to the time of food withdrawal, time of collection of sample, type of food provided and techniques applied. Apart from the diurnal pattern of stomach GOAT mRNA levels, Kirchner et al., also observed an association between stomach GOAT expression and the type of diet provided [32]. GOAT is essential for the prevention of hypoglycemia during prolonged caloric restriction or negative energy balance [32,47]. The ability of the body to maintain normal glucose levels during prolonged fasting is impaired in animals lacking the expression of GOAT [18] which is prevented by treatment with acyl Ghrelin. This suggests that this enzyme is important for survival under prolonged caloric restriction and severe starvation [18,48,49].
Availability of dietary lipids regulates the acylation of ghrelin by GOAT with the GOAT–ghrelin system being stimulated when fatty diet is eaten [18]. The GOAT–ghrelin system is activated when certain fatty foods are consumed to inform the brain about the availability of food [32] [Table/Fig-3]. Zhao has demonstrated that, in mice, GOAT is essential for survival during periods of famine and the enzyme is not essential for energy storage in times of plenty of food intake. It has been reported that mice lacking GOAT were not able to maintain normal glucose levels during prolonged caloric restriction and this impairment was prevented when mice were treated with acyl-ghrelin [18,42]. GOAT-ghrelin system is not essential for preventing hypoglycemia during prolonged energy depletion [18,48] [Table/Fig-3]. It is still not clear if there is an age-dependent effect of deficiency of GOAT on the control of hypo-glycemia in calorie-restricted conditions [48]. Caloric restriction and exogenous administration of leptin leads to an increase in GOAT mRNA in the stomach mucosa which suggests the role of GOAT mRNA levels in mediating the physiological responses to Caloric restriction and represents an adaptive response for preventing alterations in energy metabolism [48] [Table/Fig-3].
GOAT protein levels depend on the metabolic status being positively correlated with BMI and negatively with ghrelin [50] [Table/Fig-3]. Stomach GOAT mRNA levels correlates with circulating acylated-ghrelin levels in obese mice [46]. The levels are increased in morbidly obese patients and reduced in anorexic patients [40]. This indicates that, since GOAT is the only known enzyme capable of acylating ghrelin, it plays a pivotal role in development of anorexia and obesity [50].
Role of hormones: Analysis of Goat mRNA expression in animal models together with in vitro studies under different energy status suggests the influence of several hormones such as Growth-hormone-releasing hormone (GHRH), leptin, and ghrelin [50]. Leptin, GHRH and acylated-ghrelin (and not desacyl ghrelin) increases the expression of GOAT while somatostatin decreases it [40,42] [Table/Fig-3].
Leptin has been shown to increase the GOAT mRNA levels both in vitro and in vivo [40,46]. Leptin administered exogenously to fasted rats markedly increased GOAT mRNA levels during fasting very low levels of leptin prevent increased GOAT mRNA expression [40]. Also, the availability of preproghrelin mRNA is increased in fasting [20]. Study has shown that the conditions in which leptin signaling is impaired, the favorable metabolic effects mediated via the GOAT-ghrelin system is affected [20]. In the INS-1 rat insulinoma cell line, GOAT expression is down-regulated by insulin. However in primary mouse pituitary cells, insulin does not affect the expression of GOAT [32,51].
Modulation of GOAT for Controlling Obesity and T2DM
Since ghrelin and des-acyl-ghrelin appear to have opposite glucoregulatory effects, the regulation of acylation of ghrelin by GOAT seems to play a role in mediating glucose metabolism [51] by modulating insulin secretion and sensitivity [8]. Thus modulation of GOAT may be an effective approach to manage obesity, T2DM and other metabolic diseases [8]. Ablation of GOAT enhances insulin release and prevents impaired glucose tolerance in obese models which suggests that manipulation of the insulinostatic function of GOAT can optimize the amount of insulin release [8].
Inhibitors of GOAT: Stimulation of appetite by ghrelin depends on the presence of an octanoyl group. Suppressing GOAT would obstruct the appetite-stimulating action of Ghrelin and can pave a new direction to develop new drugs that obstructs the attachment of an octanoyl group to the hunger hormone and helps in countering obesity and T2DM. By increasing insulin secretion and enhancing peripheral insulin sensitivity, inhibitors of GOAT might be able to prevent diet-induced obesity and thus can be an effective therapy for T2DM and other metabolic disorders [25,38]. Studies have suggested the activity of GOAT as well as Ghrelin can be inhibited by incorporating initial five amino acids with the same sequence as those of Ghrelin [20].
Pretreatment of human islet cells with a GOAT inhibitor promotes a significant increase in insulin response and reduced blood glucose levels to a glucose challenge indicating that GOAT may catalyze acylation of ghrelin in islets [20]. The signal transduction mechanisms of the receptors of Ghrelin in pancreatic cells are distinctive, being different from those used for release of growth hormone [41]. Studies have found that when GOAT inhibitor is administered to mice, glucose tolerance test is remarkably improved which suggests that the deficiency of ghrelin prevents them from hyperglycemia induced by high fat diet [41,52].
Studies have suggested that this beneficial effect of ablation of GOAT on energy and glucose metabolism could be mediated by an increase in the desacyl ghrelin/acyl ghrelin ratio [18,32]. Attenuation of GOAT by using synthetic inhibitors increases insulin secretion and reduces body weight [38].
These findings emphasize the complexities of GOAT-ghrelin in controlling energy and glucose metabolism. The therapeutic benefits of using the Ghrelin - GOAT system and GOAT - inhibitors in treating obesity and T2DM are attractive; but, the potential scope of pharmacologic actions of such compounds is yet to be explored. Study of these modulators may provide the needed tools to map out fascinating aspects of GOAT in biology and health [37]. Exploring the biology of GOAT may bear rewards in the march towards countering obesity and diabetes. Although several interesting results have been reported to date, the research on GOAT is still in its infancy, and further researches hold the key to enhance our understanding of this enzyme and promoting its utilization for the benefit of patients with obesity, diabetes and other metabolic disorders.
Role of GOAT in octanoylatingghrelin
Regulation of GOAT mRNA expression
Conclusion
Ghrelin, a gut hormone linked to the regulation body weight and obesity, has been suggested to be such a key regulator of glucose metabolism. GOAT enzyme is responsible for mediating the physiological functions of ghrelin by generating the active form of this hormone. GOAT mRNA expression is regulated by energy balance, metabolic status and hormones like leptin, insulin, GHRH and acylated-ghrelin. The regulation of acylation of Ghrelin by GOAT seems to play a role in mediating glucose metabolism by modulating insulin secretion and sensitivity. Inhibitors of GOAT increases insulin secretion, enhance peripheral insulin sensitivity, and thereby prevent diabetes and diet induced obesity. GOAT is thus emerging as a potential therapeutic target for regulating appetite and conditions such as obesity, insulin resistance and T2DM. Although significant progress has been reported in enzymology and inhibition of GOAT in the recent years, many challenges still remain.
[1]. Global status report on noncommunicable diseases 2010. Geneva WHOTeratology 2011 [Google Scholar]
[2]. PLJ Seim I, Amorim Laura de, Walpole Carina M , Fung Jenny, Whiteside Eliza J, Lourie Rohan, Ghrelin O-acyltransferase (GOAT) is expressed in prostate cancer tissues and cell lines and expression is differentially regulated in vitro by ghrelinReproductive Biology and Endocrinology 2013 11:70 [Google Scholar]
[3]. HH Kojima M, Y Date, M Nakazato, H Matsuo, Ghrelin is a growth-hormone-releasing acylated peptide from stomachNature 1999 402:656-60. [Google Scholar]
[4]. SG Nazli Khatib, Khatib Mahnaaz, Simkhada Padam, Gaidhane Abhay, Gode Dilip, Zahiruddin Quazi Syed, Ghrelin: Ghrelin as a regulatory peptide in growth hormone secretionJ Clin Diag Res 2014 88(8):MC13-MC17. [Google Scholar]
[5]. GS Khatib MN, A Gaidhane, ZQ Syed, Role of Ghrelin in regulation of growth hormone secretion by Ghrelin-Pituitary-GH axis linkageInt J Med Sci Public Health 2014 3(4):425-29. [Google Scholar]
[6]. MA Rosalie M, V Kim, U Piet, P Axel, J Leo, Effects of acute administration of acylated and unacylated ghrelin on glucose and insulin concentrations in morbidly obese subjects without overt diabetesEur J Endocrinol 2009 161:567-73. [Google Scholar]
[7]. DG Mahalaqua Nazli Khatib, Simkhada Padam, Agho Kingsley, Gaidhane Shilpa, Saxena Deepak, B Unnikrishnan, Somatotropic and cardio-protective effects of ghrelin in experimental models of heart failure: A systematic reviewAnnals of Tropical Medicine and Public Health 2014 7(1):30-42. [Google Scholar]
[8]. CA Otto B, Role of ghrelin and leptin in the regulation of carbohydrate metabolismGhrelin Postepy Hig Med Dosw 2012 :795-98. [Google Scholar]
[9]. SS Pradhan G, Y Sun, Ghrelin: much more than a hunger hormoneCurr Opin Clin Nutr Metab Care 2013 16(6):619-24. [Google Scholar]
[10]. SP Khatib MN, D Gode, Cardioprotective effects of ghrelin in heart failure: From gut to heartHeart Views 2014 15(3):74-76. [Google Scholar]
[11]. GS Khatib MN, P Simkhada, A Gaidhane, ZQ Syed, Cardiovascular effects of ghrelin in heart failure: A systematic reviewInt J Med Sci Public Health 2014 3(6):756-63. [Google Scholar]
[12]. IT Takahashi T, T Sato, Y Nakashima, Y Nakamura, A Tsuji, M Kojima, Production of n-octanoyl-modified ghrelin in cultured cells requires prohormone processing protease and ghrelin O-acyltransferase, as well as n-octanoic acidJ Biochem 2009 146:675-82. [Google Scholar]
[13]. SP Gutierrez JA, DR Perkins, JA Willency, MD Knierman, Z Jin, DR Witcher, Ghrelin octanoylation mediated by an orphan lipid transferaseProc Natl Acad Sci USA 2008 105:6320-25. [Google Scholar]
[14]. TT Ohgusu H, M Kojima, Enzymatic characterization of GOAT, ghrelin O-acyltransferaseMethods Enzymol 2012 514:147-63. [Google Scholar]
[15]. HY Chen, ME Trumbauer, AS Chen, DT Weingarth, JR Adams, EG Frazier, Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agoutirelated proteinEndocrinology 2004 145(6):2607-12. [Google Scholar]
[16]. CB Zhang W, JY Li, H Wang, MW Mulholland, Effect of des-acyl ghrelin on adiposity and glucose metabolismEndocrinology 2008 149:4710-16. [Google Scholar]
[17]. KH Pfluger PT, S Gunnel, B Schrott, D Perez-Tilve, Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditureAm J Physiol Gastrointest Liver Physiol 2008 294:610-18. [Google Scholar]
[18]. LG Zhao TJ, RL Li, X Xie, MW Sleeman, Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted miceProc Natl Acad Sci 2010 107:7467-72. [Google Scholar]
[19]. MK Mahalaqua Nazli Khatib, Gaidhane Shilpa, Gaidhane Abhay, Zahiruddin Quazi Syed, Ghrelin for regulating appetite and energy balance: A systematic reviewNational Journal of Physiology, Pharmacy & Pharmacology 2014 4(2):101-05. [Google Scholar]
[20]. MJV Ruth C González, López Miguel, Diéguez Carlos, Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosaJ Mol Endocrinol 2008 41:415-21. [Google Scholar]
[21]. KM Hosoda H, H Matsuo, K Kangawa, Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissueBiochemical and Biophysical Research Communications 2000 279:909-13. [Google Scholar]
[22]. KP Delhanty PJ , C Gauna, B van de Zande, G Vitale, Ghrelin and its unacylated isoform stimulate the growth of adrenocortical tumor cells via an antiapoptotic pathwayJ Physiol Endocrinol Metab 2007 293:E302-09. [Google Scholar]
[23]. IA Chen CY, A Asakawa, K Fujino, I Kato, CC Chen, N Ueno, Desacyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious ratsGastroenterology 2005 129:8-25. [Google Scholar]
[24]. YH Toshinai K, Y Sun, RG Smith, A Yamanaka, T Sakurai, Y Date, Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptorEndocrinology 2006 147:2306-14. [Google Scholar]
[25]. HH Dezaki K, M Kakei, S Hashiguchi, M Watanabe, Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in betacells: implication in the glycemic control in rodents.Diabetes 2004 53:3142-51. [Google Scholar]
[26]. AM Kiewiet RM, K van der Weerd, P Uitterlinden, AP Themmen, Effects of acute administration of acylated and unacylated ghrelin on glucose and insulin concentrations in morbidly obese subjects without overt diabetesEur J Endocrinol 2009 161:567-73. [Google Scholar]
[27]. SY Delhanty P, JA Visser, A van Kerkwijk, M Huisman, Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted micePLoS One 2010 5:e11749 [Google Scholar]
[28]. PD Benso A S, F Prodam, E Gramaglia, R Granata, AJ van der Lely, E Ghigo, Metabolic effects of overnight continuous infusion of unacylated ghrelin in humansEur J Endocrinol 2012 166:911-16. [Google Scholar]
[29]. MF Gauna C, JA Janssen, PJ Delhanty, T Abribat, Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivityJ Clin Endocrinol Metab 2004 89:5035-42. [Google Scholar]
[30]. DP Gauna C, LJ Hofland, JA Janssen, F Broglio, Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytesJ Clin Endocrinol Metab 2005 90:1055-60. [Google Scholar]
[31]. GC Broglio F, F Prodam, C Gauna, G Muccioli, Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humansJ Clin Endocrinol Metab 2004 89:3062-05. [Google Scholar]
[32]. HK Kirchner H, J Holland, D Kabra, MH Tschöp, Ablation of Ghrelin O-Acyltransferase Does Not Improve Glucose Intolerance or Body Adiposity in Mice on a Leptin-Deficient ob/ob BackgroundPLoS ONE 2013 8(4):e61822 [Google Scholar]
[33]. KR Gauna C, JA Janssen, B van de Zande, PJ Delhanty, Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditionsAm J Physiol Endocrinol Metab 2007 293:E697-04. [Google Scholar]
[34]. SD Tschop M, ML Heiman, Ghrelin induces adiposity in rodentsNature 2000 407:908-13. [Google Scholar]
[35]. L Tamas, SD Horvath, Sotonyi Peter, Heiman Mark, Tschöp Matthias, Minireview: Ghrelin and the Regulation of Energy Balance—A Hypothalamic PerspectiveEndocrinology 2001 142:4163-69. [Google Scholar]
[36]. B Yang JS , Liang G, Grishin NV, Goldstein JL, Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormoneCell 2008 132:387-96. [Google Scholar]
[37]. S Martin, TRR Taylor, Hsiao Po-Yuan, Hwang Yousang, Zhang Pingfeng, Dai Lixin, Architectural Organization of the Metabolic Regulatory Enzyme Ghrelin-O-AcyltransferaseThe Journal of Biological Chemistry 2013 288:32211-28. [Google Scholar]
[38]. US Mohan H, Discovery of ghrelin o-acyltransferaseEndocr Dev 2013 :2516-24. [Google Scholar]
[39]. KB Lim CT, A Grossman, M Korbonits, The expression of ghrelin O-acyltransferase (GOAT) in human tissuesEndocr J 2011 58:707-10. [Google Scholar]
[40]. C-CJ Gahete MD, M Hergueta-Redondo, AJ Martinez-Fuentes, RD Kineman, G Moreno-Bueno, RM Luque, A novel human ghrelin variant (In1- ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: potential pathophysiological relevancePLoS One 2011 6:e23302 [Google Scholar]
[41]. KD Ghrelin function in insulin release and glucose metabolismEndocr Dev 2013 25:135-43. [Google Scholar]
[42]. HK Amparo Romero, Heppner Kristy, Pfluger Paul T, Tschöp Matthias H, Nogueiras Ruben, GOAT: the master switch for the ghrelin system?Eur J Endocrinol 2010 163:1-8. [Google Scholar]
[43]. J Yang, T-J Zhao, J Goldstein, M Brown, Inhibition of Ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptidesProctl Natl Acad Sci 2008 105(31):10750-55. [Google Scholar]
[44]. HY Taylor MS, PY Hsiao, JD Boeke, PA Cole, Ghrelin O-acyltransferase assays and inhibitionMethods Enzymol 2012 514:205-28. [Google Scholar]
[45]. X Zhu, Y Cao, K Voogd, DF Steiner, On the processing of proghrelin to ghrelinJ Biol Chem 2006 281(50):38867-70. [Google Scholar]
[46]. VM González CR, M López, C Diéguez, Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosaJ Mol Endocrinol 2008 41(6):415-21. [Google Scholar]
[47]. KMH Chun-Xia Yi, T Paul, Pfluger. The GOAT-Ghrelin System Is Not Essential for Hypoglycemia Prevention during Prolonged Calorie RestrictionPLoS One 2012 7(2):e32100 [Google Scholar]
[48]. CY The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restrictionPLoS One 2012 7:e32100-772. [Google Scholar]
[49]. CX Yi, KM Heppner, H Kirchner, J Tong, M Bielohuby, BD Gaylinn, The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restrictionPLoS One 2012 7(2):e32100 [Google Scholar]
[50]. HT Stengel M , U Elbelt, T Pauline, A Anne, K Peter, The ghrelin activating enzyme ghrelin-O-acyltransferase (GOAT) is present in human plasma and expressed dependent on body mass indexPeptides 2013 43:13-19. [Google Scholar]
[51]. LY An W, G Xu, J Zhao, X Xiang, L Ding, J Li, Modulation of ghrelin O acyltransferase expression in pancreatic isletsCell Physiol Biochem 2010 26:707-16. [Google Scholar]
[52]. HY Barnett BP, MS Taylor, H Kirchner, PT Pfluger, Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitorScience 2010 330:1689-92. [Google Scholar]