COPD is a chronic condition of progressive and irreversible airflow limitation with inflammation in response to noxious particles or gases [1]. Oxidative stress is considered as one of the pathogenic mechanisms in COPD, which causes excessive tissue damage. Oxidative stress is indicated by increased lipid peroxidation product MDA and other oxidants, with concomitant decrease in antioxidants like Vitamin C, E, catalase, superoxide dismutase, etc. [2]. Oxidative damage seen in COPD is due to the exposure to exogenous oxidants from cigarette smoke, tobacco, biomass fuel burning, dust, pollution, etc., as well as endogenously produced oxidants from activated inflammatory cells. By measuring the level of these substances (oxidants and antioxidants) in blood, we can easily determine the magnitude of oxidative stress in any pathological conditions, like COPD [3].
Along with oxidative stress, low BMI and malnutrition are equally associated with COPD each having independent effect on mortality. Improvement in severity as seen after nutritional support and weight gain indicates the need of nutritional management, but still results are not conclusive [4]. Oxidative stress is also suggested to be associated with alteration in BMI and malnutrition [5].
Although, impaired oxidative stress and malnutrition have been reported in COPD, we lack adequate data from Nepal where malnutrition, air pollution (indoor and outdoor), excessive tobacco consumption, etc. are major public health problem and are also the predictors of COPD [6-7]. Thus, the combined effect of a fore mentioned prevailing conditions in the outcome of COPD, for example oxidative stress, are still obscure. In this study, we aimed to determine the burden of oxidative stress in COPD and assess their nutritional status.
Materials and Methods
Institutional ethical clearance was taken for this study and signed informed consent was obtained from every participating subject or from their guardian if necessary. This cross-sectional study was conducted from August 2010 to July 2011 where we enrolled 100 cases of COPD from emergency and medical ward. Simultaneously, age, sex and occupation (mainly farmers, housewives and drivers) matched 100 control subjects without COPD and meeting inclusion criteria were selected for comparison.
Inclusion Criteria
For COPD cases, individual coming to emergency and those admitted in medical ward due to COPD were included with their consent and/or from their attendee. For controls, walking individuals not having COPD irrespective of other conditions and not taking any medications were selected with their consent. Controls were matched in age, sex and occupation mainly farmers, housewives and drivers. Presence of other chronic diseases except of respiratory origin was neglected during control selection.
Exclusion Criteria
Exclusion criteria for selection of COPD cases were those not willing to participate in the study, cases with any other conditions except COPD and COPD cases taking any known antioxidant or vitamin supplementation. Exclusion criteria for selection of controls were those not willing to participate in the study, those under medication for respiratory disease, those having recent hospital visit (less than a week) for chronic condition and known case of respiratory disease.
Definition
Oxidant/antioxidant imbalance, also called oxidative stress, was defined by an increase in MDA (a lipid peroxidation product, marker of oxidative stress) with concomitant decrease in antioxidants (Vitamin C, E and RBCC).
Assessment of Nutritional Status
Data on height and weight were taken from patient medical record file in COPD cases. In non-COPD controls, height was measured by stadiometer (Seca 216 Accu-Hite) and weight was measured by standardized balance, finally BMI was calculated in both groups. The base line characteristics like age, sex, occupation, smoking habit and BMI, in both case and control groups were recorded. Nutritional Assessment was done by measuring BMI as given by World Health Organization (WHO) [8], i.e., underweight (BMI ≤ 18.5 Kg/m2), normal weight (BMI 18.5-24.9 Kg/m2), overweight (BMI 25.0-29.9 Kg/m2) and obese (BMI ≥ 30 Kg/m2). Along with BMI, pre-standardized MNA [9] tool was also used to asses nutritional status among COPD cases. This questionnaire based tool was developed by Guigoz et al., [9] to identify hospitalized elderly persons, who are malnourished or at risk of malnutrition. This tool consists of questionnaires which were summed up and a final score was interpreted as, score of 0-7 = malnutrition, score of 8-11 = risk of malnutrition and score of 12-14 = normal nutritional status.
Determination of Biochemical Parameters
Fasting plasma sample was used for determination of MDA [10], Vitamin C [11] and Vitamin E [12] whereas red blood cell lysate was used for determination of catalase activity [13]. Whole blood sample was used for Haemoglobin estimation [14]. Oxidative stress marker and antioxidants were measured in both COPD cases and non-COPD controls for comparison. COPD cases were further grouped into different categories by their BMI and oxidative stress was assessed in each group and compared with the normal BMI group.
All the chemicals and reagents required for estimation of biochemical parameters were purchased from India based branch of Sigma Aldrich Co. LLC. USA. Spectrophotometric measurements were made on Shimadzu UV-1201 Spectrophotometer. All the centrifugation was done in Remi research centrifuge model R-23, ISO 9001:2000 certified.
Statistical Analysis
Data entry and statistical analysis was conducted using Statistical Package for Social Sciences, version 11.0 (SPSS, Inc. Chicago, IL, USA). In all analysis, statistical significance was defined as p < 0.05 (two tailed). Chi square test was applied for categorical variable. Student t-test was applied for comparison of means between two groups. ANOVA was applied for comparison between groups followed by Bonferroni post hoc analysis. Comparison of quantitative variables was done by pearson correlation method.
Results
This study consists of 100 COPD cases and 100 controls without COPD irrespective of other chronic conditions except of respiratory origin, matched in age, sex and occupation (farmers, housewives and drivers). The baseline characteristics of cases and controls are summarized in [Table/Fig-1]. All COPD cases were more than in their fifties and most of them were smokers (76%) and few non-smokers (16%). There was no significant difference in age, sex and occupation between cases and controls p > 0.05 but there was highly significant difference in BMI and smoking habit between cases and controls (p < 0.001) as shown in [Table/Fig-1]. Female predominance (64%) over males (36%) was seen in having the disease. Most of the COPD cases were found to be underweight (48%), i.e., BMI ≤ 18.5 kg/m2. COPD was higher in housewives (52%) followed by farmers (26%).
Baseline characteristics of COPD cases and non-COPD controls
| Characteristics | Cases n = 100 | Controls n = 100 | x2 statistics p-value |
|---|
| Age (years)* | 51% | 62% | 0.12 |
| 50-69 |
| 70 and above | 49% | 38% |
| Gender* | 36% | 36% | 1.00 |
| Male |
| Female | 64% | 64% |
| Occupation* | 7% | 13% | 0.26 |
| Driver |
| Farmer | 26% | 18% |
| Housewife | 52% | 49% |
| Other | 15% | 20% |
| BMI, kg/m2* | 48% | 15% | <0.001 |
| ≤18.5 (underweight) |
| 18.5-24.9 (normal weight) | 30% | 56% |
| 25-29.9 (over weight) | 13% | 16% |
| ≥30 (obese) | 9% | 13% |
| Smoking habit* | 76% | 46% | <0.001 |
| Smoker |
| Non-smoker | 16% | 32% |
| Past smoker | 8% | 22% |
*Number and percentage corresponds to each other in each case
[Table/Fig-2] shows the comparison of lipid peroxidation marker MDA and antioxidants like Vitamin C, E and RBCC between COPD cases and non-COPD controls. Student’s t-test was used for comparison of means between cases and controls. The mean level of oxidative stress marker MDA was significantly increased with decrease in the levels of antioxidants like Vitamin E and catalase in COPD cases as compared to control (p < 0.001). The difference in the level of Vitamin C was not significant (p = 0.19) between two groups. Data also shows significantly low BMI in COPD cases as compared to controls (p < 0.001).
Comparison of oxidative stress marker MDA and antioxidants between COPD cases and non-COPD controls
| Parameters | Cases (n = 100) | Controls n = 100 | p-value |
|---|
| Plasma MDA (nmol/ml) | 8.53 ± 1.78 | 5.35 ± 1.72 | <0.001 (95% CI, 2.68–3.66) |
| Plasma Vitamin C (mg/dl) | 0.69 ± 0.26 | 0.74 ± 0.25 | 0.19 (95% CI, 0.023–0.12 |
| Plasma Vitamin E (mg/dl) | 0.64 ± 0.32 | 1.10 ± 0.42 | <0.001 (95% CI, 0.35–0.56) |
| RBC Catalase (U/gm Hb) | 32.59 ± 18.43 | 64.87 ± 23.47 | <0.001 (95% CI, 26.39–38.16) |
| BMI (Kg/m2) | 20.67 ± 5.28 | 23.34 ± 4.70 | <0.001 (95% CI, −5.17 − −2.27) |
Values are expressed as mean ± SD. CI: Confidence Interval
[Table/Fig-3] shows nutritional status in COPD cases according to score obtained from MNA tool. BMI along with MNA tool was used to assess nutritional status. According to this, 55% of the cases were found to be malnourished and most of them were underweight (46%). Risk of malnutrition (18%) as well as normal nutrition (27%) was also obvious finding among COPD cases.
Bar diagram showing nutritional assessment in COPD by MNA tool and BMI

[Table/Fig-4] shows one-way between group ANOVA to analyse the impact of BMI on the level of MDA and antioxidants (Vitamin C, E and catalase). Subjects with COPD were divided into four groups by their BMI. There was statistically significant difference in the mean level of MDA, Vitamin C and E for different BMI groups (p < 0.05), but the difference in the level of catalase was not significant. Here normal weight was taken as reference and other groups were compared with it during post-hoc analysis.
Comparison of nutritional status as described by BMI and associated oxidative stress among COPD cases
| Parameters | BMI (Kg/m2) | p- value |
|---|
| Underweight (≤18.5) (n = 48) | Normal weight (18.5–24.9) (n = 30) | Overweight (25.0–29.9) (n = 13) | Obese (≥30) (n = 9) |
|---|
| Plasma MDA (nmol/ml) | 9.36 ± 1.40 | 7.46 ± 1.54 | 7.73 ± 1.98 | 8.81 ± 1.89 | <0.001 |
| Plasma Vitamin C (mg/dl) | 0.59 ± 0.23 | 0.82 ± 0.29 | 0.77 ± 0.19 | 0.67 ± 0.21 | 0.002 |
| Plasma Vitamin E (mg/dl) | 0.49 ± 0.15 | 0.89 ± 0.44 | 0.69 ± 0.09 | 0.53 ± 0.19 | <0.001 |
| RBC catalase (U/gm Hb) | 31.01 ± 12.47 | 35.35 ± 24.43 | 34.64 ± 4.74 | 28.96 ± 1.87 | 0.945 |
Values are expressed as mean ± SD
Comparison within the group by Bonferroni post-hoc analysis showed highly and significantly lower level of Vitamin C (p = 0.001) and Vitamin E (p = 0.001) in underweight subjects as compared to normal weight subjects. Plasma MDA level was significantly elevated (p < 0.001) in underweight subjects as compared to normal weight subjects. Others were not significant.
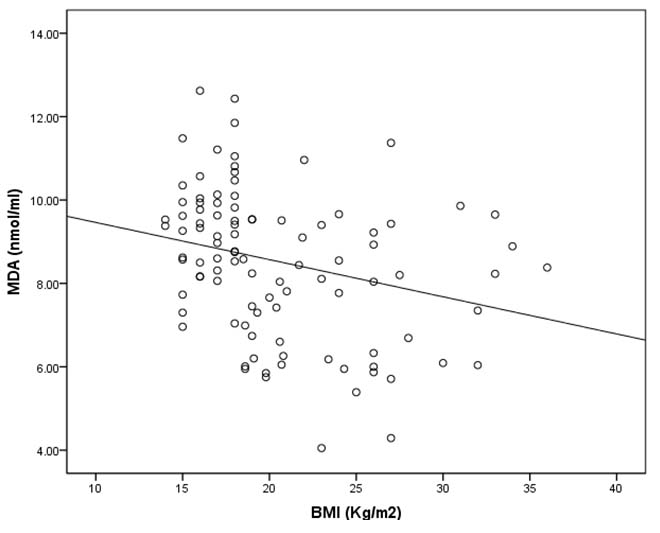
[Table/Fig-5] shows the bivariate correlation analysis of lipid peroxidation product MDA and BMI in COPD cases. This shows negative and inverse correlation (Pearson’s correlation coefficient r = −0.27, p = 0.008) between MDA and BMI, which was statistically significant.
Scatter plot showing negative and inverse correlation between BMI and MDA level in COPD cases
Discussion
According to this study, subjects with COPD are in increased burden of oxidative stress compared to controls without COPD. Our study is supported by various other studies, which have shown elevated level of lipid peroxidation product like MDA, ethane, pentane, etc. and decreased level of plasma antioxidants like Vitamin C, E in COPD and in healthy smokers as compared with non-COPD healthy non-smokers [15–16]. Elevated level of MDA and decreased level of antioxidants like glutathione peroxidase and catalase was reported in COPD cases as compared to controls in a study done in 2007 [17]. Studies show that intake of antioxidants like Vitamin E in diet improves lung function which suggest that vitamin supplementation can be another therapeutic approach in management of the disease [3].
BMI has been used for assessing malnutrition [18] but recent study suggests MNA tool can also be effective in assessing malnutrition [9]. Both of these tools have good prognostic value in assessing mortality in COPD [4,17–18]. Another finding of our study is that most of the COPD cases (55%) were malnourished and underweight (48%) as well as at the risk of malnutrition (18%). Higher rate of malnutrition in this study may be due to existing malnutrition in Nepal. Studies suggest that eating and swallowing difficulties, severe loss of appetite due to anorexiogenic effects of Tumour Necrosis Factor alpha (TNFα), high metabolic rate, loss of visceral proteins, and loss of fat free mass together with oxidative stress can contribute to malnutrition and subsequent loss of weight in COPD [19].
In addition, studies show that malnutrition occurs in 20%–70% cases of COPD and is more in severe cases [20]. Similar to our finding, a study done in France also reported malnutrition in 60% of the COPD cases among which 39% were normal weight, which is much higher than our study (9%). Author in this study suggests the need of nutritional support during COPD [19]. Similarly, a study by Giron et al., reported malnutrition (BMI < 20 kg/m2) in 38% and 40% under the risk of malnutrition among COPD cases [21]. Our study and few other studies shows that malnutrition and risk of malnutrition can be present even in normal BMI among COPD cases [19, 22]. Most of the subjects in our study were underweight (48%) and it is speculated that leaner subjects are more susceptible to COPD and other respiratory diseases irrespective of exposure to traditional risk factors. This tendency can cause reduced intake of dietary antioxidants, which can contribute to the oxidative stress. The low calorigenic state seen in COPD is not only responsible for lowering body weight but also for lipid oxidation and oxidative stress, which can exacerbate the condition [23]. A prospective study reports 24% of COPD cases were underweight, 46% normal weight and 29% overweight at baseline; but during follow up, underweight subjects had bad prognosis which was independent of smoking habit [24]. This indicates the importance of studying nutritional status and other associated issues in COPD.
We classified COPD cases into different categories by their BMI and assessed their oxidant/antioxidant status. Underweight COPD cases were found to have high burden of oxidative stress than normal weight subjects. Similar to our finding, a study done in 2007 reports that the decrease in BMI was associated with increased oxidative stress with negative and inverse correlation between BMI and MDA. Author suggested that oxidative stress has apoptotic potential, which leads to muscle atrophy and results in low BMI making leaner subjects more susceptible to COPD [20]. In contrast to our finding, a study done in 2004 reports no correlation between BMI and MDA in COPD cases and also there was no significant difference in MDA level in different BMI groups among COPD [25]. Increase in MDA could be one of the causes of loss of body weight since it is a marker of cell damage and cell damage can reduce the cell mass as long term effect, however, more study is required for conclusive results.
Another interesting finding of our study is the female predominance in bearing disease. Increase in tobacco consumption in women and combined effect of malnutrition along with indoor air pollution due to biomass fuel burning in low and middle-income countries, disease is now more prevalent in women [26]. In our study, also most of the females were housewives and were continuously exposed to heavy smoke generating medium for cooking, so this may be one of the causes of female predominance.
Our study also shows non-smokers with COPD. Although smoking is an important risk factor for COPD but there are significant numbers of never smokers developing COPD ranging between 25% and 45% worldwide. Exposure to other types of smoke, dust, indoor and outdoor pollution, genetic make-up, environmental condition, childhood infection, etc., are the major determinants in developing COPD in majority of non-smokers and those who are not exposed to risk factors [27].
Limitations
Despite of striking findings, our study has few limitations.
Cross sectional design of our study could not reveal the long-term effect of oxidative stress, malnutrition and reduced BMI in COPD.
The effect of other medication in COPD cases during hospital stay could not be totally nullified.
Effect of other chronic conditions in oxidative stress in controls without COPD, also could not be totally nullified.
Conclusion
Our study has shown high burden of oxidative stress in COPD when compared with matched controls without COPD. Malnutrition was frequently present in COPD and was associated with high burden of oxidative stress, than in relatively nourished COPD cases. Presence of COPD in females in high frequency and even in non-smokers was unique finding of our study.
Recommendations
One implication of our study is the use of both BMI and MNA tool which could provide more accurate measure of malnutrition.
Another implication is that the findings of this study may light on the need of nutritional support and antioxidant therapy for better outcome, further guiding the success of therapy and necessary interventions.
Furthermore, in this study we have used simple, accurate and inexpensive methods for determination of oxidants and antioxidants as well as for assessing nutritional status. This methods can be useful in resource limited setting for different kinds of clinical studies.
*Number and percentage corresponds to each other in each case
Values are expressed as mean ± SD. CI: Confidence Interval
Values are expressed as mean ± SD