Case 1
A 22-year-old male patient reported to the Department of Conservative Dentistry and Endodontics with a chief complaint of pain in the upper left front teeth region associated with swelling for one month. He gave history of trauma around 10 y back and noticed discoloration of teeth with time but no history of treatment. Intraoral examination revealed there were no carious teeth. Intraoral periapical radiograph (IOPA) revealed large periapical radiolucency in relation to upper left central and lateral incisor. Root apex of tooth 21 revealed blunderbuss type apex. Electric pulp testing (EPT) was done to check the vitality of the tooth which confirmed that the teeth 21 and 22 were non vital and tender on percussion. Endodontic treatment was taken up first and then periapical surgery was planned for treatment of defect. Access opening done in teeth 21 and 22, root canals were prepared using step back technique till an apical size of # 70 and #60 in relation to teeth 21 and 22 respectively. 5.25% sodium hypochlorite solution (Novo Dental Product Pvt Ltd, Mumbai, India) was used as irrigation agent during biomechanical preparation. Calcium hydroxide was given as intracanal medicament and recalled after a month.
PRF Preparation
Before proceeding the cases ethical clearance was obtained from the university ethical committee for all the cases. Choukroun’s protocol had been followed for the preparation of PRF. Just prior to surgery, 8 ml intravenous blood was drawn from the antecubital vein and was collected in a 10-ml sterile tube without anticoagulant. The tube was centrifuged immediately at 3,000 rotations per minute for 10 min. This allows the structured fibrin clot in the middle of the tube between the red blood cells at the down most and acellular plasma layer which lacks platelets at the top. A natural and progressive polymerization which occurs during centrifugation results the formation of PRF. PRF can be separated easily from red blood cells layer using a sterile tweezers and scissors from the tube. PRF was then transferred onto a sterile dapen dish and stored in refrigerator.
Surgical Procedure
The extraoral antisepsis was carried out using iodine solution. A full thickness mucoperiosteal flap was reflected under local anaesthesia by a sulcular incision starting from the distal of the tooth 11 to distal of the tooth 23. A large periapical defect was seen with complete loss of labial cortical plate in relation to the left central and lateral incisor. The lesion measured 15 mm x 20 mm x 16 mm corresponding to the length, width, and depth of the lesion. Thorough tissue curettage was done at the defect site followed by irrigation using sterile saline solution. Mineral trioxide aggregate (ProRoot MTA; Dentsply, Tulsa Dentals, OK, USA) was used as the root end filling material upto 5 mm in tooth 21 and tooth 22 was obturated using gutta-percha with lateral condensation technique. Endoflas FS (Sanlor, Colombia) was used as a sealer. The PRF prepared and stored prior to surgery was mixed with HA bone graft (Sybograf(®) by sprinkling the graft material over the PRF gel and together the mixture was placed into the intrabony defect. The mucoperiosteal flaps were repositioned and secured in place by giving simple interrupted suture using 3-0 non absorbable black silk surgical suture.
Patient was kept under the antibiotic (amoxycillin) and analgesic (Diclofenac) coverage. The chlorhexidine gluconate solution (0.2%) was prescribed as mouth rinse for a period of five days. Suture removal was done after one week and the healing was uneventful. Patient was reviewed at three months, six months, one year, and two year period during which there were no symptoms of pain, discomfort and inflammation. Routine intraoral examinations and professional plaque control had been done during these review periods. Resorbed area replaced with new bone at the end of two years radiographically [Table/Fig-1].
Case 2
A 19-year-old female patient reported to the Department of Conservative Dentistry and Endodontics with a complaint of pain in the upper left front tooth region associated with pus discharge for the past one week. She gave history of trauma around six years and history of orthodontic treatment. There were no carious teeth on intraoral examination. Intra oral radiograph revealed large periapical radiolucency in relation to upper left central and lateral incisor. EPT was done to check the vitality of the teeth which confirmed that the teeth were non vital and pain on percussion. Endodontic treatment was taken up first and then periapical surgery was planned for treatment of defect. Access opening done in teeth 21 and 22, root canals were prepared using step back technique upto an apical size of # 70 and #60 in relation to teeth 21 and 22 respectively. 5.25% sodium hypochlorite solution (Novo Dental Product Pvt Ltd, Mumbai, India) was used as irrigation agent during biomechanical preparation. Calcium hydroxide was given as intra canal medicament and recalled after a month.
Just prior to periapical surgery 10 ml blood was collected from the patient and PRF was prepared. Surgical procedures were followed as mentioned in case 1. The lesion measured 10 mm x 12 mm x 8 mm corresponding to the length, width, and depth of the lesion. Thorough tissue curettage was done at the defect site followed by irrigation using sterile saline solution. PRF was mixed HA bone graft (Sybograf(®) by sprinkling the graft material over the PRF gel and together the mixture was placed inside the bone cavity. Teeth 21 and 22 were obturated using gutta-percha with lateral condensation technique. Endoflas FS (Sanlor, Colombia) was used as a sealer. Patient was reviewed at three months, six months and one year period during which there were no symptoms of pain, discomfort and inflammation [Table/Fig-2].
Case 3
A 24-year-old male patient reported to the Department of Conservative Dentistry and Endodontics with a complaint of pain in the lower front teeth region associated with pus discharge for two weeks. He gave history of trauma around seven years and no history of treatment. On intraoral examination there were no carious teeth and discoloured 41. IOPA radiograph revealed large periapical radiolucency in relation to lower incisors. EPT was done to check the vitality of the teeth which confirmed that the teeth 41, 42, 31 and 32 were non vital and the teeth 31 and 41 were tender on percussion. Teeth 32, 41 & 42 were grade I mobile and tooth 31 was grade II mobile. Endodontic treatment was taken up first and then periapical surgery was planned for treatment of defect. Access opening done in teeth 31, 32, 41 and 42, root canals were prepared using step back technique till an apical size of # 50. 5.25% sodium hypochlorite solution (Novo Dental Product Pvt Ltd, Mumbai, India) was used as irrigation agent during biomechanical preparation. Calcium hydroxide was given as intra canal medicament and recalled after a month.
Just prior to periapical surgery 10 ml blood was collected from the patient and PRF was prepared. Surgical procedures were followed as mentioned in case 1. The lesion measured 10 mm x 20 mm x 8 mm corresponding to the length, width, and depth of the lesion. Thorough tissue curettage was done at the defect site followed by irrigation using sterile saline solution. PRF was mixed HA bone graft (Sybograf(®) by sprinkling the graft material over the PRF gel and together the mixture was placed inside the bone cavity. Teeth 31, 32, 41 and 42 were obturated using gutta-percha with lateral condensation technique. Endoflas FS (Sanlor, Colombia) was used as a sealer. Patient was reviewed at three months, six months and one year period during which there were no symptoms of pain, discomfort and inflammation [Table/Fig-3].
Discussion
The primary goal of dental treatment is the maintenance of the natural dentition in health and for optimum comfort, function, and esthetics. Orthograde root canal therapy should be the first option for the treatment of all inflammatory periapical lesions which has 85% of success rate [1]. Nevertheless failure after orthograde root canal treatment needs surgical intervention. Periapical Surgery has some limitations, such as is an invasive procedure, requires skilled and experienced operator and also has psychological impact on the patient [2,3]. Nevertheless, periapical surgery remains the last resort when orthograde treatment fails or is not possible. Recent years Platelet rich fibrin (PRF) becomes a biological revolution in dental field. PRF contains and releases different growth factors that stimulate bone and soft tissue healing [4]. Growth factors are actively participating in tissue repair mechanism such as angiogenesis, chemotaxis, cell proliferation, extracellular matrix deposition, and remodelling. Autologous platelet storage via PRF is an easy, cost-effective way to obtain high concentrations of growth factors for tissue healing. The PRF production protocol attempts to accumulate platelets and released cytokines in a fibrin clot. Currently available clinical evidence suggests that healing rate of surgical sites enhanced with PRF is upto two to three times higher than normal surgical sites [5].
Hydroxyapatite (HA) bone grafts have excellent bone conductive properties and permit outgrowth of osteogenic cells from existing bone surfaces into the adjacent bone material [6]. HA bone grafts well tolerated clinically, owing to lack of organic contents it does not develop allergic reaction. This case report is an attempt to present a successful clinical outcome and the healing potential of the combination of PRF and HA bone graft in large periapical lesions.
Body’s healing potential can be maximized by local application of growth factors and host modulating agents, which further promotes periapical tissue regeneration and healing. Platelet Derived Growth Factor (PDGF) and Transforming Growth Factor beta (TGF-β) are two main growth factors, which encourages healing of soft tissue and bone through stimulation of collagen production to ameliorate wound strength and initiation of callus formation [7]. PDGF synchronize the migration, proliferation and survival of mesenchymal cell lineages. Among all cytokines the TGF-beta contains the most powerful fibrosing agent. It induces enormous synthesis of matrix molecules such as fibronectin and collagen-I either by fibroblasts or osteoblasts. Nevertheless the regulation mechanism is complex and is pondered as an inflammation regulator through its capacity to induce fibrous cicatrization. Various studies have proved that platelets have alpha-granules, contain these growth factors [8,9]. The stem cells present in apical tissues are attracted by these growth factors [10]. Platelets are the rich source of growth factors so Platelet Rich Plasma (PRP) and PRF are used to deliver these growth factors.
PRP was developed by Whitmen in 1997 and considered as a first generation platelet concentrate. PRP was introduced for the purpose of deliver the concentrated growth factors such as PDGF, TGF-ß, and insulin like growth factor- 1 (IGF-1) to the surgical site. This enriches the natural blood clot in order to accomplish quick wound healing and stimulate bone regeneration [11]. The human blood clot contains 95% red blood cells (RBCs), 5% platelets, less than 1% white blood cells (WBCs), and many fibrin strands on the other hand PRP contains 4% RBCs, 95% platelets, and 1% WBCs. The PRP production protocol necessitates blood collection with anticoagulant, two stage centrifugation, and artificial polymerization of the platelet concentrate using clot activators such as calcium chloride and bovine thrombin [12].
PRP has been used clinically to stimulate bone regeneration however its real efficacy is questionable [12]. Schlegel et al., suggested that PRP mediate only the early aspects of bone regeneration and its long term efficacy remains questionable, and the expected benefits are moderate [13].
The PRF is considered as a second-generation platelet concentrate. Choukroun developed the PRF making protocol in 2001. The PRF is produced without adding an anticoagulant, bovine thrombin, or calcium chloride is not needed for platelet activation and fibrin polymerization [14]. The homogenous fibrin network formed from centrifugation is more highly coherent than natural fibrin clots. PRF composed of a fibrin matrix polymerized in a tetra molecular structure, with inclusion of platelet, cytokines, circulating stem cells and leucocytes [15].
The autologous growth factors released by PRF in a gradual manner for at least one week and up to 28 d [16]. The homologous organization of fibrin network is formed during centrifugation by natural and slow polymerization. The anticoagulant absence leads to massive platelet activation in the test tube. A progressive polymerization incorporates intrinsic circulating cytokines in the fibrin meshes. This configuration increases the lifespan of these cytokines, only at the time of initial cicatricial remodelling they are released and used [17]. PRF also has a more durable and stronger effect than PRP [14] The elastic nature of PRF membrane acts like a fibrin bandage which accelerates the healing of wound edges. The use of PRF has numerous advantages over PRP [Table/Fig-4]. The use of biological platelet extracts (PRF, PRP) in healing of inflammatory lesions have been reported by various authors [Table/Fig-5].
HA has shown successful periodontal regeneration in periapical defects. The combination of HA with PRF resulted in greater pocket depth reduction, defect fill and gain in clinical than PRF used alone [25]. This was the reason behind the selection of HA. HA could maintain the space for tissue regeneration and it could enhance the effects of PRF. HA is an osteoconductive material and exerts its effects in defect areas thus induces bone regeneration. Nevertheless without a blood clot or angiogenic factors, bone grafts alone are unlikely to be capable of promoting periapical wound healing [26]. As the host’s own biologic product the blood clot is indispensable in tissue wound healing and acts as better space filler than all bone grafting materials. The benefits of using a combiration of PRF which is plate with bone grafts are improved handing properties of graft materials, graft stabilzation, hemostasis, promoting wound healing, bone growth and maturation.
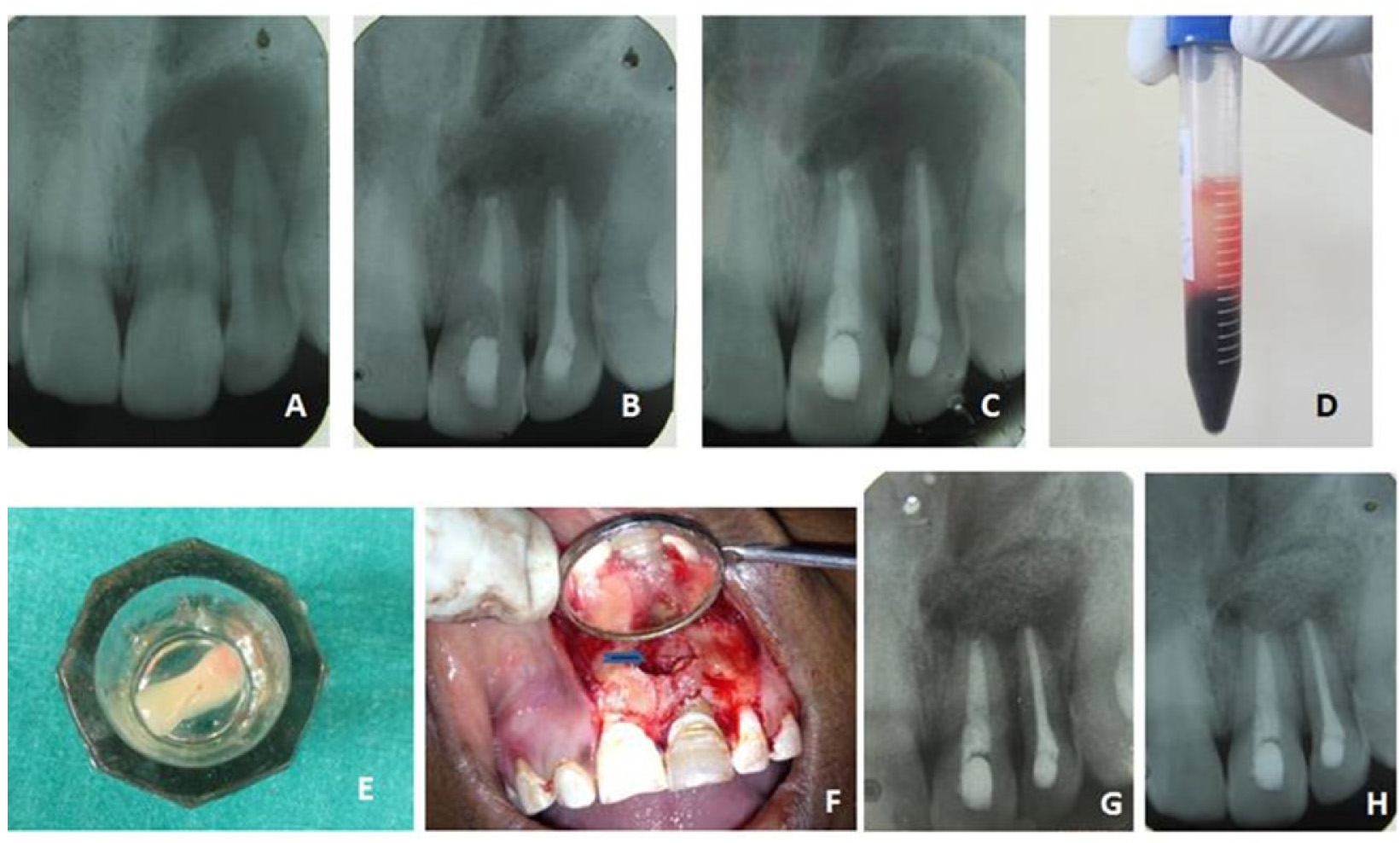
Clinical picture of case 1
A - Pre operative IOPA radiograph showing large periapical radiolucency in relation to 21 & 22.
B - MTA apical plug in 21
C - After complete obturation of 21 and 22
D - PRF preparation
E - PRF layer isolated from tube
F - PRF and HA bone graft placed inside bone cavity
G - 6 month review
H - 2 year review
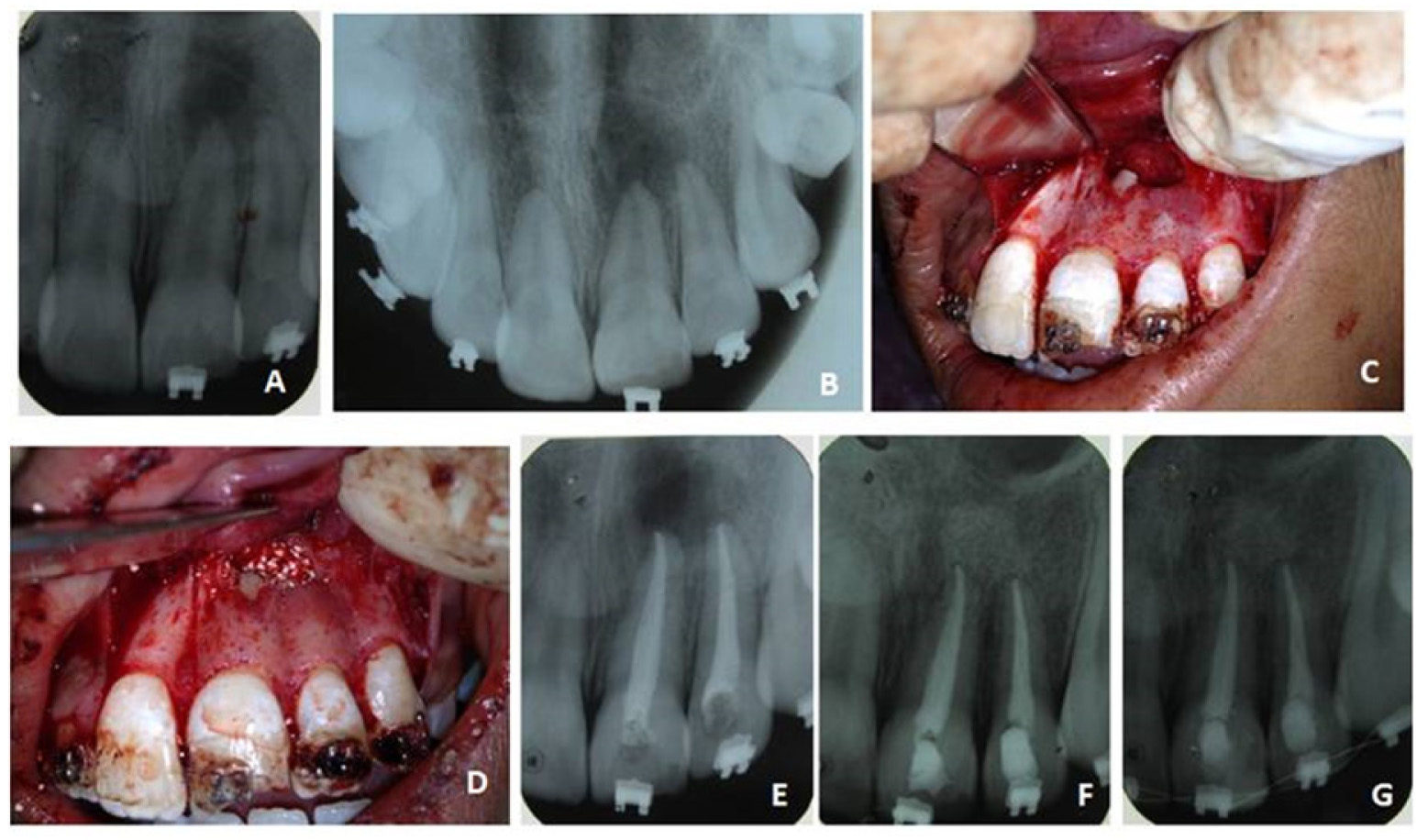
Clinical picture of case 2
A, B - Pre operative IOPA and Occlusal radiograph showing periapical radiolucency in relation to 21 & 22.
C - Bone cavity after through curettage
D - PRF and HA bone graft placed inside bone cavity
E - After obturation of 21 and 22
F - 6 month review
G - 1 year review
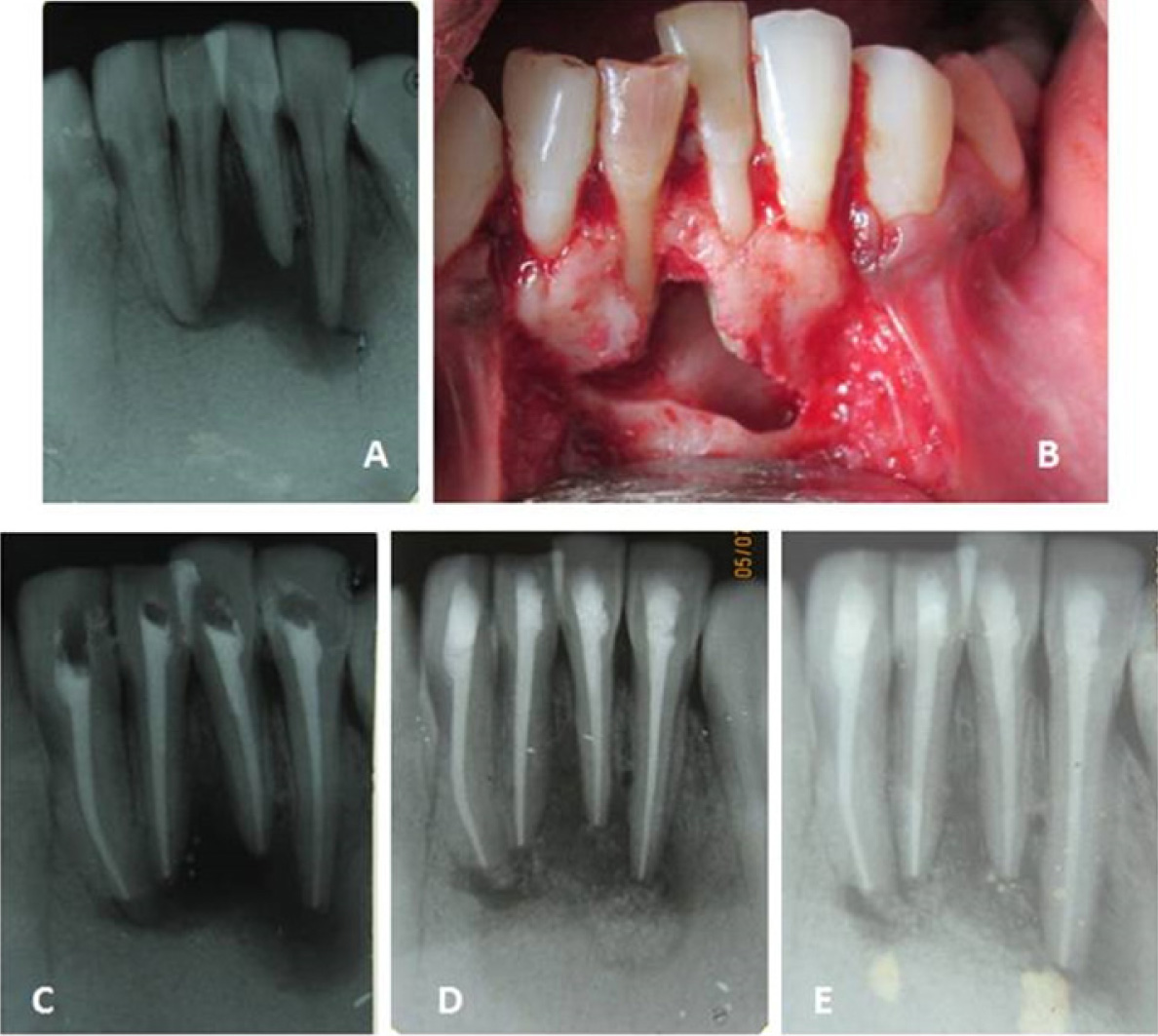
Clinical picture of case 3
A - Pre operative IOPA radiograph showing large periapical lesion in relation to lower incisor teeth
B - Bone cavity after through curettage
C - After obturation of 21 and 22
D - 6 month review
E - 1 year review
PRF and PRP- The advantages and the disadvantages. Saluja et al., (2011) [18]
| Advantages of Platelet-rich fibrin over platelet-rich plasma | Disadvantages of Platelet-rich fibrinNo |
|---|
| No biochemical handling of blood | Amount available is low, becuase of autologous blood |
| Simplified and cost effective process and use of bovine thrombin and anticoagulants not required | Quick handling of blood is needed, immediately after collection |
| Favorable healing due to slow polymerization | |
| More efficient cell migration and proliferation | |
| PRF has supportive effect on immune system | |
| PRF helps in haemostasis | |
Reported cases of periapical surgery with PRF and PRP
| Author | Year | Teeth | Material | Follow up |
|---|
| Goyal L [19] | 2014 | 22 | PRF & alloplastic bone graft | 12 months |
| Bhandari et al., [20] | 2013 | 11 and 21 | PRF & Osseograft | 14 months |
| Singh et al., [21] | 2013 | 15 cases of upper anteriors | PRF | 6 months |
| Shivashankar et al., [6] | 2013 | 11 and 12 | PRF & HA graft | 24 months |
| Jayalakshmi et al., [22] | 2012 | 21 and 22 | PRF and β-TCP | 12 months |
| Vaishnavi et al., [23] | 2011 | 20 cases in a study | PRP & HA | 12 months |
| Demiralp et al., [24] | 2004 | 11 and 21 | PRP & TCP | 12 months |
Conclusion
To conclude, that the use of PRF and HA bone graft might accelerate the bone regeneration. However histological studies are more appropriate to confirm the bone regeneration. Randomized controlled trials and long term clinical studies are required to know the effects of this combination.
[1]. LM Lin, GTJ Huang, PA Rosenberg, Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healingJ Endod 2007 33(8):908-16. [Google Scholar]
[2]. EJ Neaverth, HA Burg, Decompression of large periapical cystic lesionsJ Endod 1982 8(4):175-82. [Google Scholar]
[3]. TL Walker, MS Davis, Treatment of large periapical lesions using cannulization through the involved teethJ Endod 1984 10(5):215-20. [Google Scholar]
[4]. NE Carlson, RB Roach, Platelet-rich plasma Clinical applications in DentistryJ Am Dent Assoc 2002 133(10):1383-86. [Google Scholar]
[5]. RE Marx, ER Carlson, RM Eichstaedt, SR Schimmele, JE Strauss, KR Georgeff, Platelet-rich plasma: growth factor enhancement for bone graftsOral Surg Oral Med Oral Pathol Oral Radiol Endod 1998 85:638-46. [Google Scholar]
[6]. VY Shivashankar, DA Johns, S Vidyanath, G Sam, Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesionJ Conserv Dent 2013 16:261-64. [Google Scholar]
[7]. JD Bashutski, HL Wang, Periodontal and endodontic regenerationJournal of Endodontics 2009 35(3):321-28. [Google Scholar]
[8]. XE Dereka, CE Markopoulou, IA Vrotsos, Role of growth factors on periodontal repairGrowth Factors 2006 24(4):260-67. [Google Scholar]
[9]. WV Giannobile, RA Hernandez, RD Finkelman, Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis Journal of Periodontal Research 1996 31(5):301-12. [Google Scholar]
[10]. M Torabinejad, M Turman, Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case reportJournal of Endodontics 2011 37(2):265-68. [Google Scholar]
[11]. E Soffer, JP Ouhayoun, F Anagnostou, Fibrin sealants and platelet preparations in bone and periodontal healingOral Surg Oral Med Oral Pathol Oral Radiol Endod 2003 95:521-28. [Google Scholar]
[12]. RE Marx, ER Carlson, RM Eichstaedt, SR Schimmele, JE Strauss, KR Georgeff, Platelet-rich plasma: Growth factor enhancement for bone graftsOral Surg Oral Med Oral Pathol Oral Radiol Endod 1998 85:638-46. [Google Scholar]
[13]. KA Schlegel, K Donath, S Rupprecht, De novo bone formation using bovine collagen and platelet-rich plasmaBiomaterials 2004 25(23):5387-. [Google Scholar]
[14]. DM Dohan, J Choukroun, A Diss, Platelet-rich fibrin (PRF): a secondgeneration platelet concentrate. Part I: technological concepts and evolutionOral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 2006 101(3):E37-44. [Google Scholar]
[15]. M Toffler, N Toscano, D Holtzclaw, MD Corso, DD Ehrenfest, Introducing Choukroun’s Platelet Rich Fibrin (PRF) to the Reconstructive Surgery MilieuImplant Dent 2009 1:21-30. [Google Scholar]
[16]. L He, Y Lin, X Hu, Y Zhang, H Wu, A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitroOral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 2009 108(5):707-13. [Google Scholar]
[17]. DM Dohan, J Choukroun, A Diss, Platelet-rich fibrin (PRF): a secondgeneration platelet concentrate. Part II: platelet-related biologic featuresOral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 2006 101(33):E45-50. [Google Scholar]
[18]. H Saluja, V Dehane, U Mahindra, Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeonsAnn Maxillofac Surg 2011 1:53-57. [Google Scholar]
[19]. L Goyal, Clinical effectiveness of combining platelet rich fibrin with alloplastic bone substitute for the management of combined endodontic periodontal lesionRestor Dent Endod 2014 39(1):51-55. [Google Scholar]
[20]. S Bhandari, T S Ashwini, R Naik, T Bandiwadekar, S Makandar, Regenerative periapical surgery: A case reportDent Hypotheses 2013 4:61-66. [Google Scholar]
[21]. S Singh, A Singh, S Singh, R Singh, Application of PRF in surgical management of periapical lesionsNatl J Maxillofac Surg 2013 4:94-99. [Google Scholar]
[22]. KB Jayalakshmi, S Agarwal, MP Singh, BT Vishwanath, A Krishna, R Agrawal, Platelet-rich fibrin with beta-tricalcium phosphate-a noval approach for bone augmentation in chronic periapical lesion: a case reportCase Rep Dent 2012 2012:902858 [Google Scholar]
[23]. C Vaishnavi, B Mohan, L.L Narayanan, Treatment of endodontically induced periapical lesions using hydroxyapatite, platelet-rich plasma, and a combi- nation of both: An in vivo studyJ Conserv Dent 2011 2:140-46. [Google Scholar]
[24]. B Demiralp, HG Keçeli, M Muhtaroğullar, A Serper, B Demiralp, K Eratalay, Treatment of periapical inflammatory lesion with the combination of platelet-rich lasma and tricalcium phosphate: A case reportJ Endod 2004 30:796-800. [Google Scholar]
[25]. AR Pradeep, P Bajaj, NS Rao, E Agarwal, SB Naik, Platelet-rich fibrin combined with a porous hydroxyapatite graft for the treatment of three-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trialJ Periodontol 2012 83:1499-507. [Google Scholar]
[26]. L Laurell, J Gottlow, Guided tissue regeneration updateInt Dent J 1998 48:386-98. [Google Scholar]